POLYMERS What is a polymer many Poly unit







- Slides: 13

POLYMERS What is a polymer?

many » Poly = __________ unit » mer = __________ many units » Polymer = _______________ one » Mono = _________ » Monomer = _________ one unit Let’s break it down

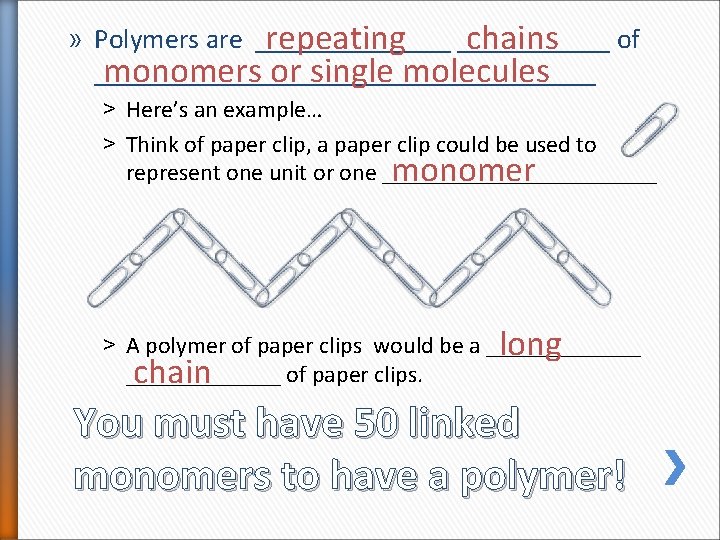
» Polymers are _______ of repeating ______ chains __________________ monomers or single molecules ˃ Here’s an example… ˃ Think of paper clip, a paper clip could be used to represent one unit or one ____________ monomer ˃ A polymer of paper clips would be a _______ long _______ of paper clips. chain You must have 50 linked monomers to have a polymer!

» To make the chain, many links or “-mers” chemically (monomers) are _________ hooked together in a process Polymerization called: ______________ Polymerization is defined as the chemical _______ reaction that joins many _________ to make a Monomers ___________ Polymer What is it called?

» A ___________ is a Catalyst substance that helps the chemical reaction occur fast enough without ________ undergoing any permanent chemical change itself. » (kind of like a taxi cab!) More Vocab!

small » Polymers are ____ because cannot see them with our we ______ unaided eyes but in the world of _____, atoms they are very ____. large How big are they?

» » » » » PLASTIC _______________________ Disposable diapers contain- Sodium Polyacrylate Clothes polyvinyl Elmer’s _______ glue contain ________ acetate resin Shoes Tortoise shells wood _______ Proteins, _________, carbohydrates lipids (fat molecules), DNA Erasers cornstarch _________ Examples

Paper » ________ » CDs » _____________ egg cartons » Plastic bags » ____ plastic soda bottles » Paper napkins » Disposable plates (plastic, styrofoam or paper) » Pieces of wood Some things made out of polymers

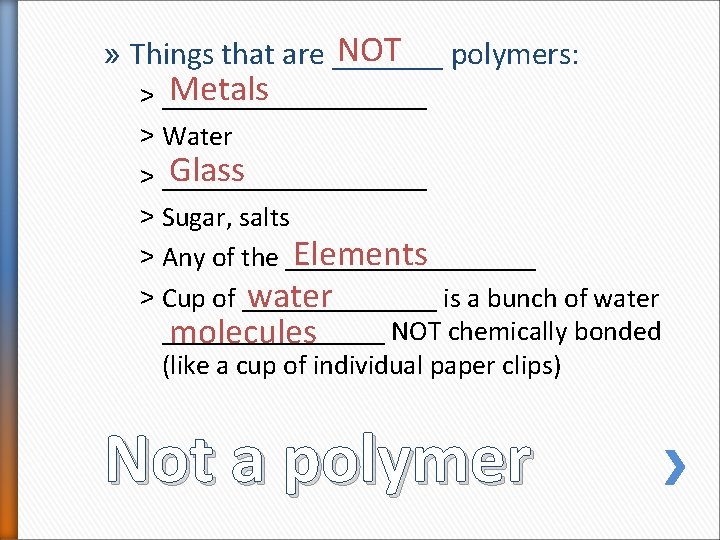
NOT polymers: » Things that are _______ Metals ˃ __________ ˃ Water Glass ˃ __________ ˃ Sugar, salts Elements ˃ Any of the _________ ˃ Cup of _______ is a bunch of water ________ NOT chemically bonded molecules (like a cup of individual paper clips) Not a polymer

» Polymers are important in part because they can act in so many different _________ ways. » Scientists can make polymers that have properties different __________. » Some hold ______ - _____ CDs music liquids » Some hold ______ - like soda diapers bottles, cups, disposable _____! What’s the big deal?

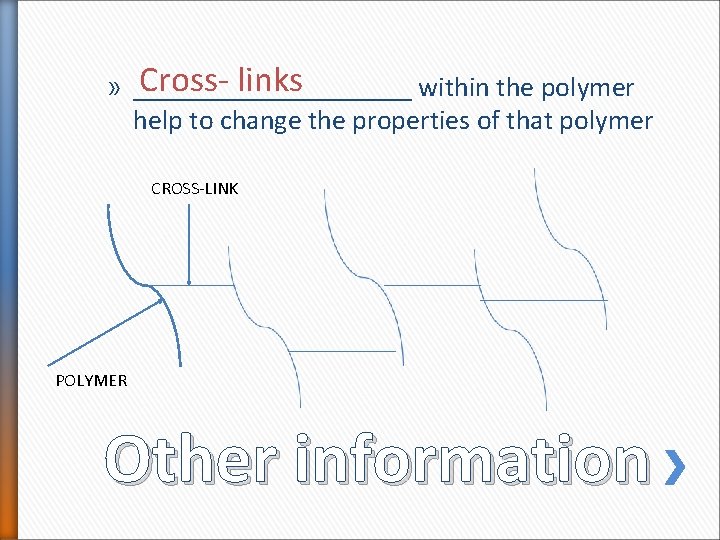
Cross- links » __________ within the polymer help to change the properties of that polymer CROSS-LINK POLYMER Other information

» _____-_______ Non Newtonian Fluids- do NOT follow the rules that Sir Newton discovered that most liquids follow ˃ Non-Newtonian fluids act like both a ______ solid liquid and a ______! ˃ Non-Newtonian fluids react to _______ STRESS with INCREASED VISCOSITY ˃ Examples: Quicksand + ________ Cornstarch + ________ Non-Newtonian

» ______Viscosity how slow a liquid _____. flows HIGHER » The SLOWER a liquid flows the ______ its VISCOSITY. » Viscosity results from the strength of attraction ______ between the particles of the liquid… ˃ “slow as molasses” Examples: Honey- has a _____ HIGH viscosity Water- has a _____ viscosity LOW Oils- have a _____ HIGH viscosity Viscosity