Polymers long chains of AMINO ACIDS arranged in


- Slides: 27

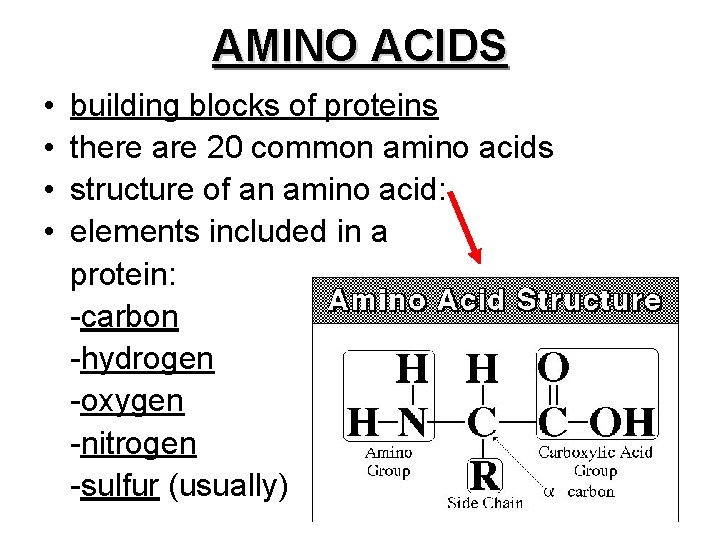
• Polymers (long chains) of AMINO ACIDS – arranged in specific sequence – linked by PEPTIDE BONDS – range in length from a few to 1000+

AMINO ACIDS • • building blocks of proteins there are 20 common amino acids structure of an amino acid: elements included in a protein: -carbon -hydrogen -oxygen -nitrogen -sulfur (usually)

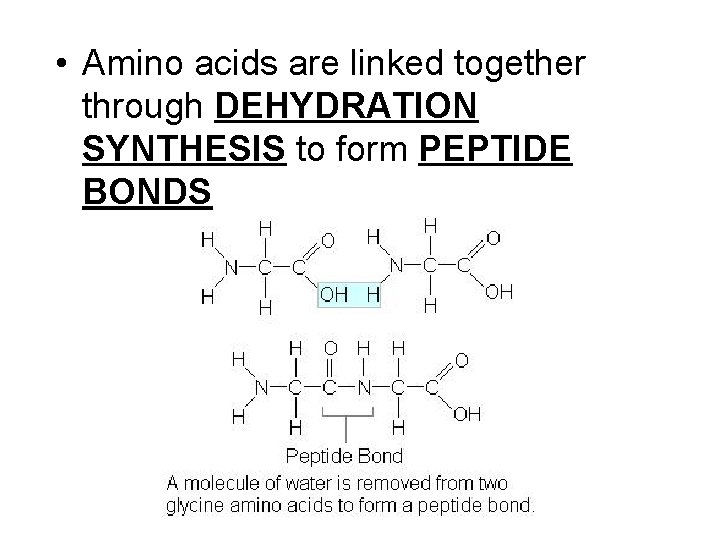
• Amino acids are linked together through DEHYDRATION SYNTHESIS to form PEPTIDE BONDS

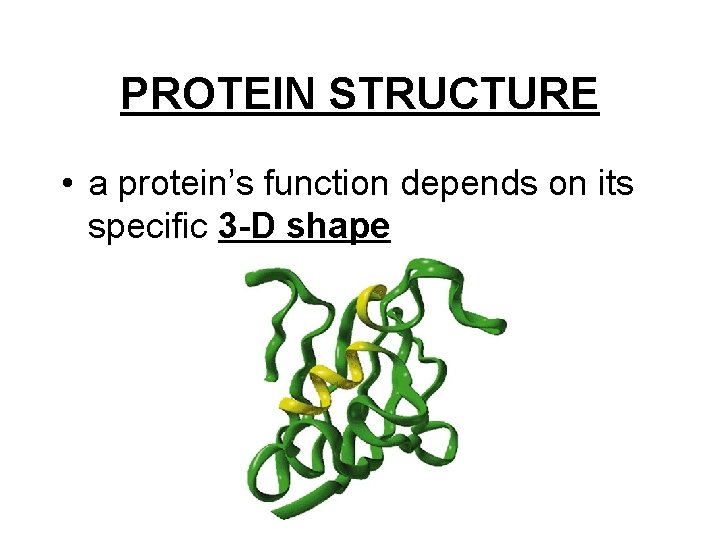
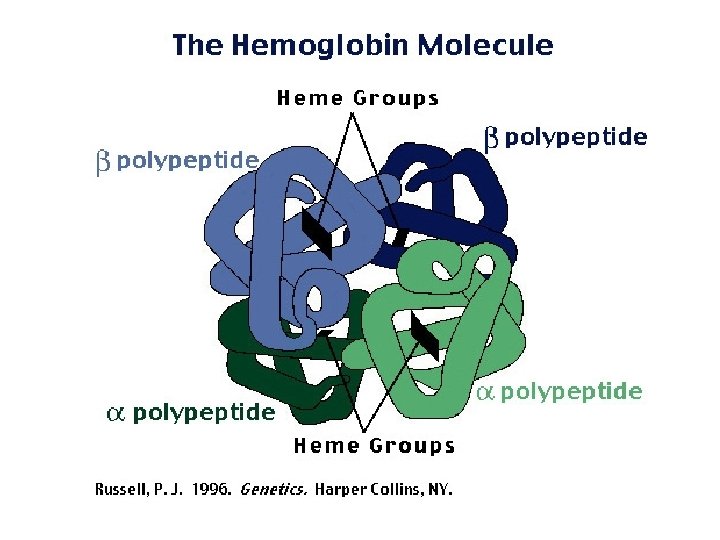
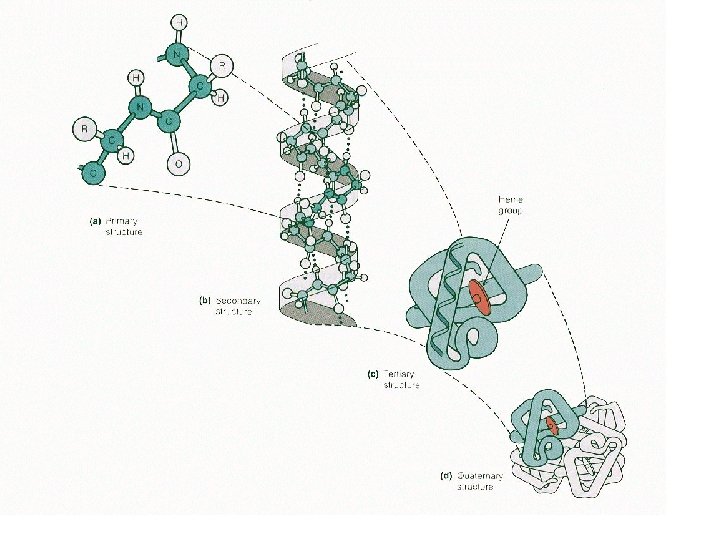
PROTEIN STRUCTURE • a protein’s function depends on its specific 3 -D shape



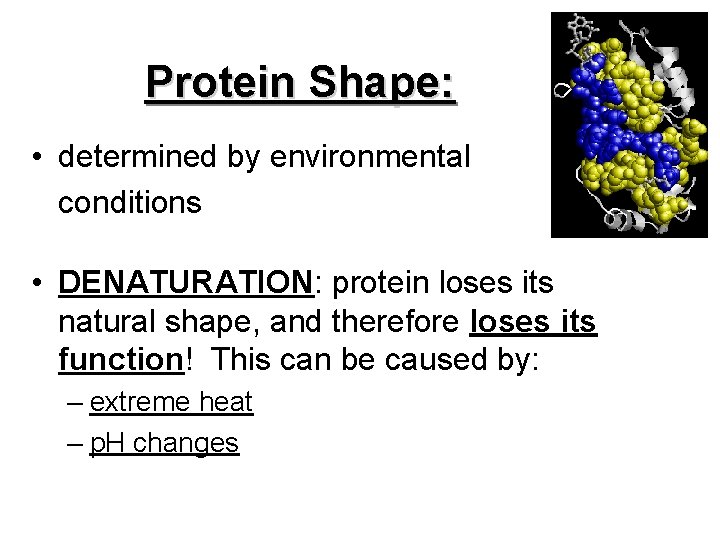
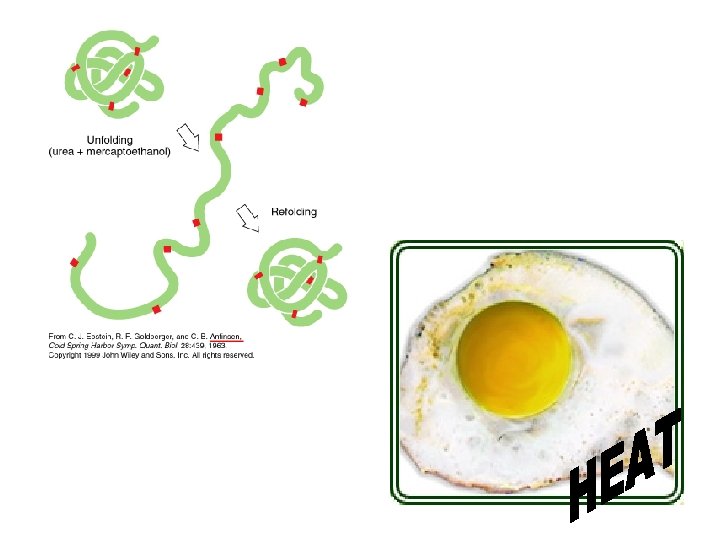
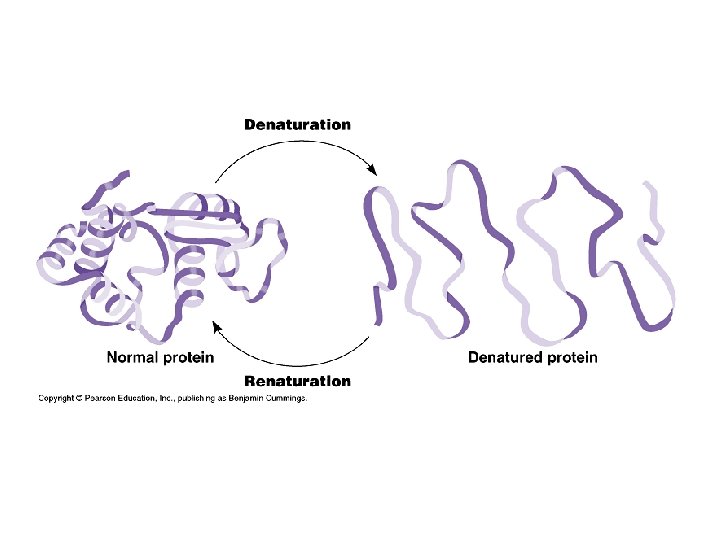
Protein Shape: • determined by environmental conditions • DENATURATION: protein loses its natural shape, and therefore loses its function! This can be caused by: – extreme heat – p. H changes


Functions of Proteins • structural support (e. g. hair, nails) • signaling (e. g. hormones)

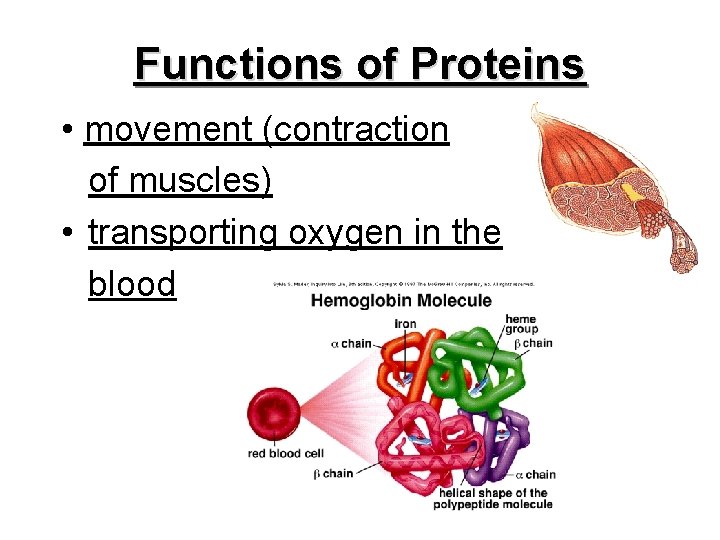
Functions of Proteins • movement (contraction of muscles) • transporting oxygen in the blood

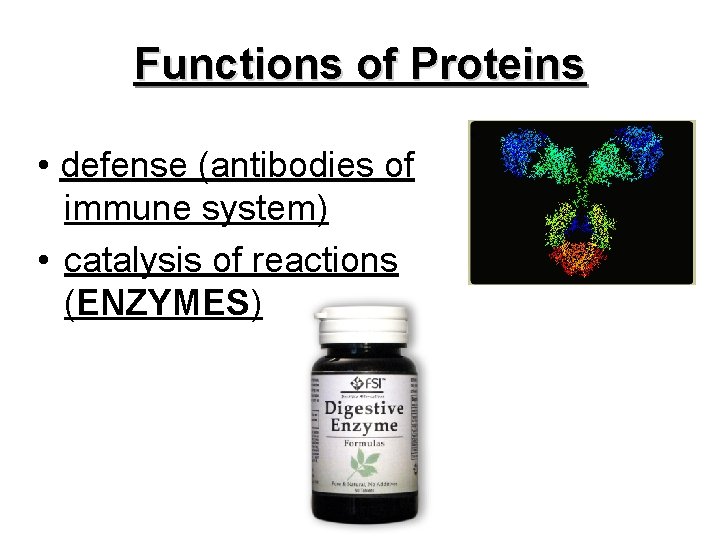
Functions of Proteins • defense (antibodies of immune system) • catalysis of reactions (ENZYMES)

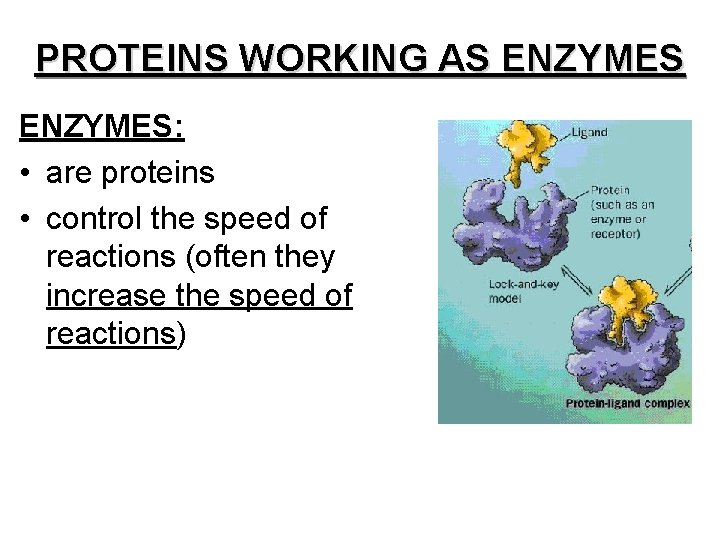
PROTEINS WORKING AS ENZYMES: • are proteins • control the speed of reactions (often they increase the speed of reactions)

• are not changed or “used up” by a reaction; can be used over and over

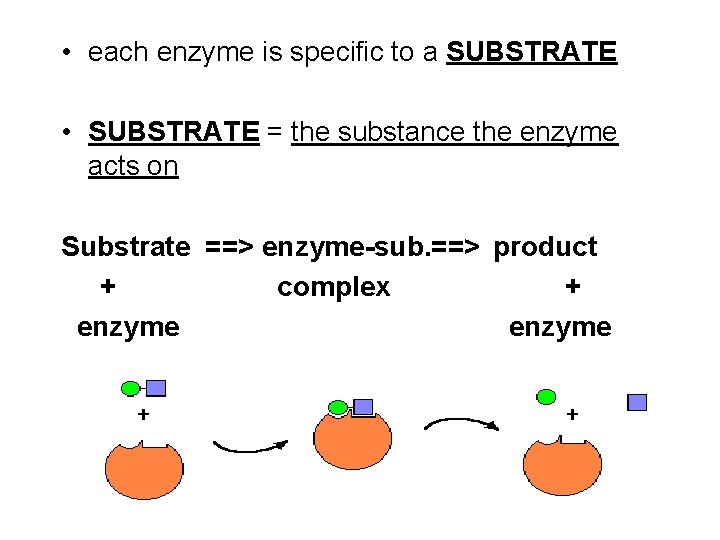
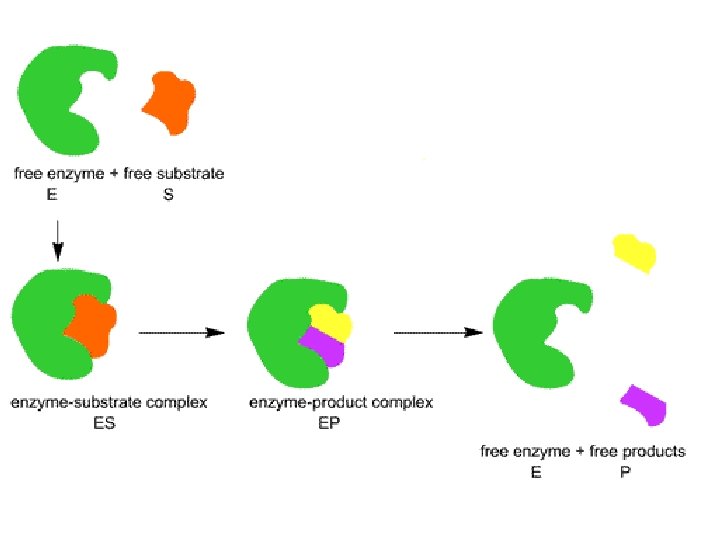
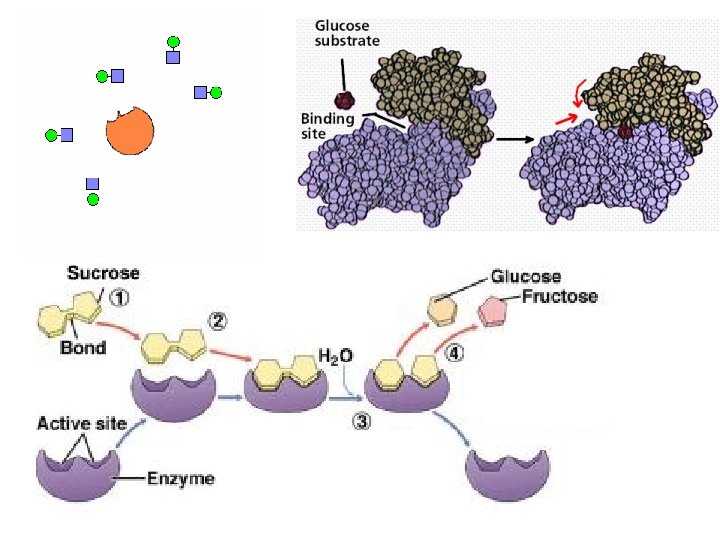
• each enzyme is specific to a SUBSTRATE • SUBSTRATE = the substance the enzyme acts on Substrate ==> enzyme-sub. ==> product + complex + enzyme


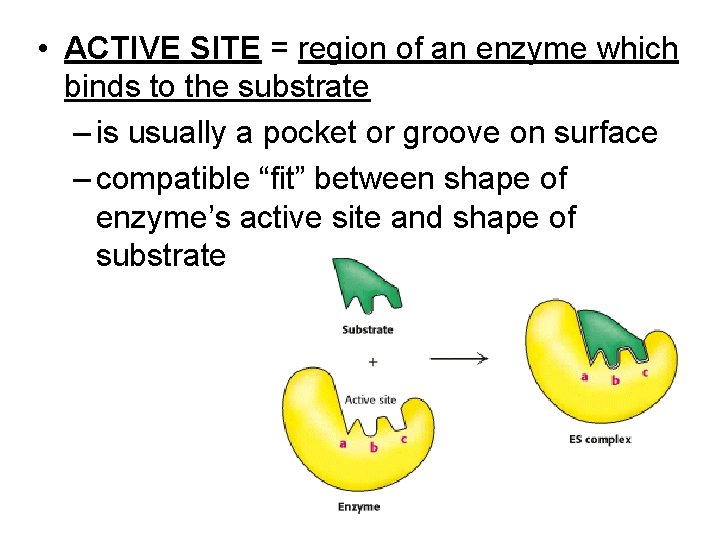
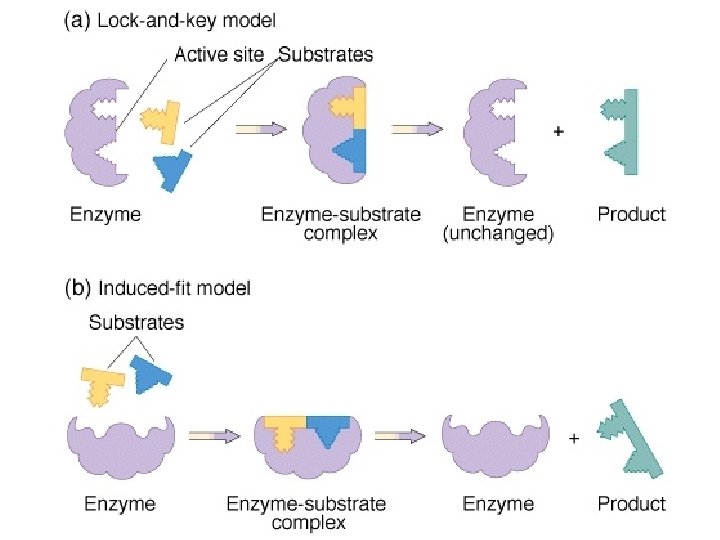
• ACTIVE SITE = region of an enzyme which binds to the substrate – is usually a pocket or groove on surface – compatible “fit” between shape of enzyme’s active site and shape of substrate



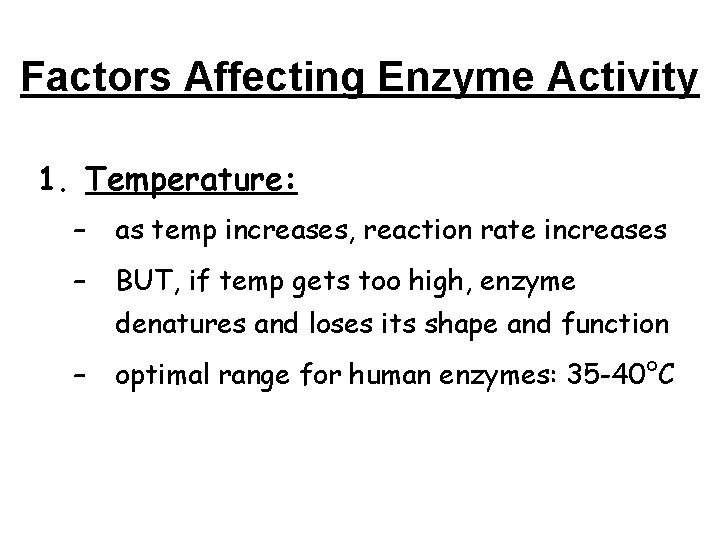
Factors Affecting Enzyme Activity 1. Temperature: – as temp increases, reaction rate increases – BUT, if temp gets too high, enzyme denatures and loses its shape and function – optimal range for human enzymes: 35 -40°C


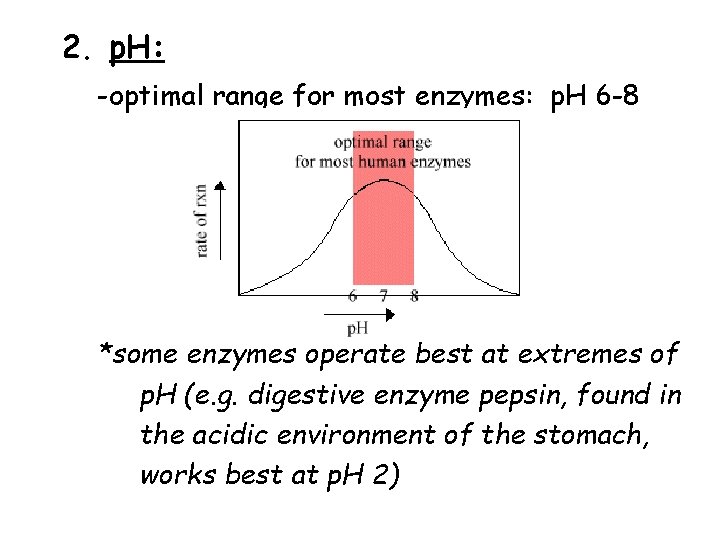
2. p. H: -optimal range for most enzymes: p. H 6 -8 *some enzymes operate best at extremes of p. H (e. g. digestive enzyme pepsin, found in the acidic environment of the stomach, works best at p. H 2)

• nucleic acids store and transmit hereditary information • Two types of nucleic acids: 1. DNA 2. RNA

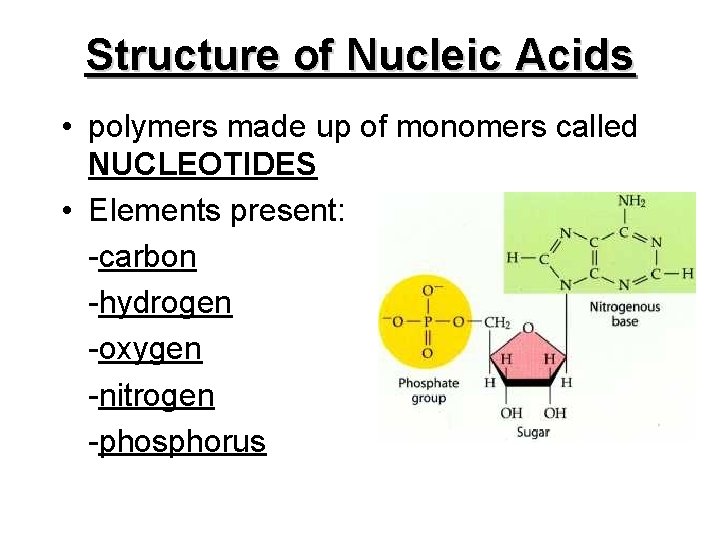
Structure of Nucleic Acids • polymers made up of monomers called NUCLEOTIDES • Elements present: -carbon -hydrogen -oxygen -nitrogen -phosphorus

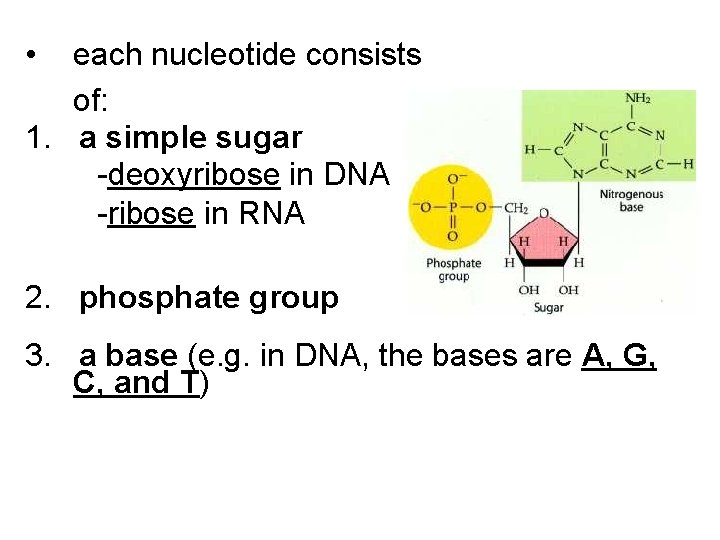
• each nucleotide consists of: 1. a simple sugar -deoxyribose in DNA -ribose in RNA 2. phosphate group 3. a base (e. g. in DNA, the bases are A, G, C, and T)

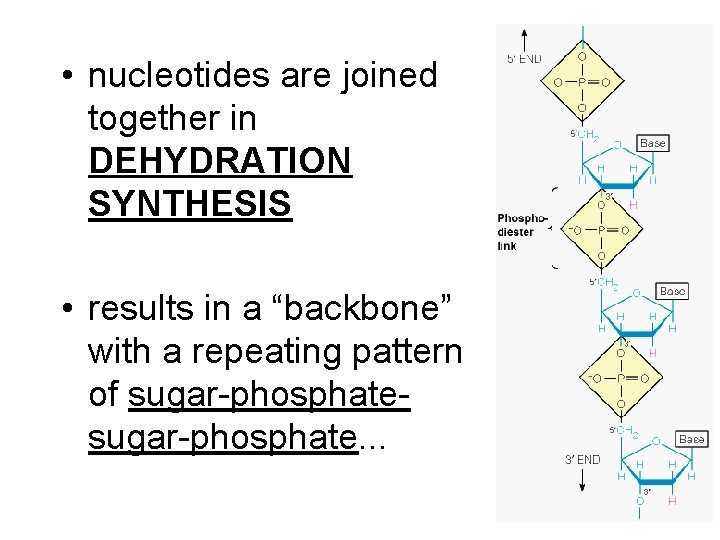
• nucleotides are joined together in DEHYDRATION SYNTHESIS • results in a “backbone” with a repeating pattern of sugar-phosphate. . .

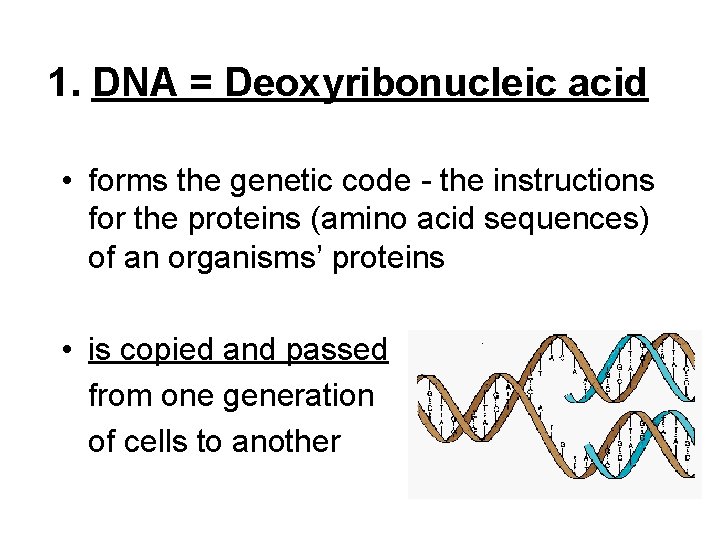
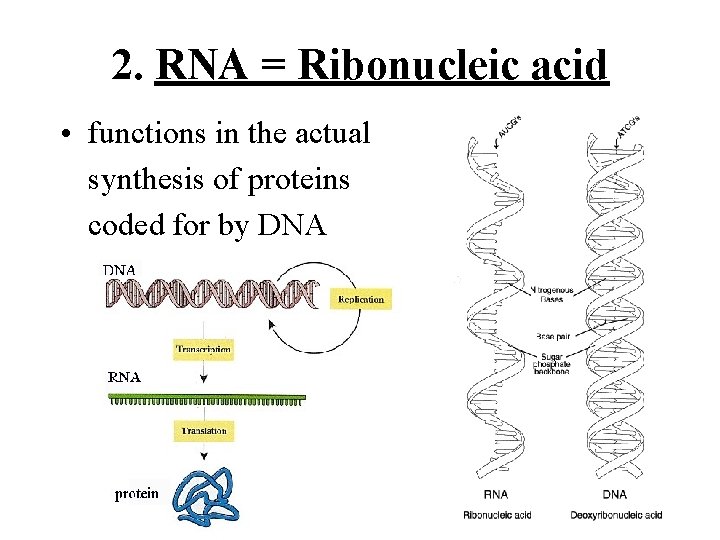
1. DNA = Deoxyribonucleic acid • forms the genetic code - the instructions for the proteins (amino acid sequences) of an organisms’ proteins • is copied and passed from one generation of cells to another

2. RNA = Ribonucleic acid • functions in the actual synthesis of proteins coded for by DNA