POLYMERS CONTENTS Prior knowledge Types of polymerisation Addition

- Slides: 13

POLYMERS CONTENTS • Prior knowledge • Types of polymerisation • Addition polymerisation • Polymerisation of propene • Condensation polymerisation • Peptides • Check list

POLYMERS Before you start it would be helpful to… • know the functional groups found in organic chemistry • know the arrangement of bonds around carbon atoms • recall and explain electrophilic addition reactions of alkenes

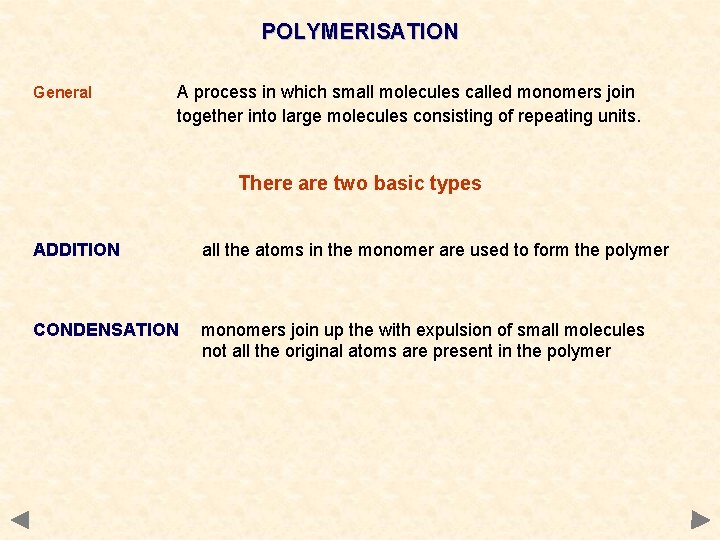
POLYMERISATION General A process in which small molecules called monomers join together into large molecules consisting of repeating units. There are two basic types ADDITION all the atoms in the monomer are used to form the polymer CONDENSATION monomers join up the with expulsion of small molecules not all the original atoms are present in the polymer

POLYMERISATION OF ALKENES ADDITION POLYMERISATION Preparation Many are prepared by a free radical process involving high pressure, high temperature and a catalyst. The catalyst is usually a substance (e. g. an organic peroxide) which readily breaks up to form radicals whichinitiate a chain reaction. Another famous type of catalyst is a Ziegler-Natta catalyst (named after the scientists who developed it). Such catalysts are based on the compound Ti. Cl 4. Properties Physical varied by changing the reaction conditions (pressure, temperature etc). Chemical have chemical properties based on the functional groups in their structure. poly(ethene) is typical; it is fairly inert as it is basically a very large alkane. This means it is resistant to chemical attack and non-biodegradable.

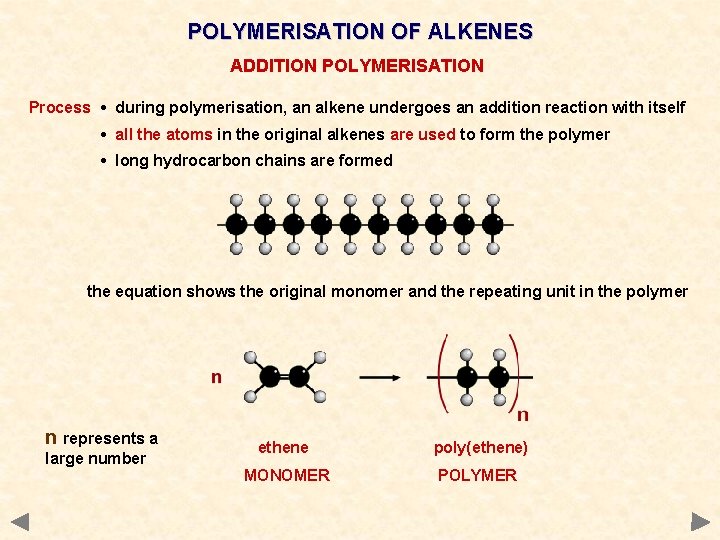
POLYMERISATION OF ALKENES ADDITION POLYMERISATION Process • during polymerisation, an alkene undergoes an addition reaction with itself • all the atoms in the original alkenes are used to form the polymer • long hydrocarbon chains are formed the equation shows the original monomer and the repeating unit in the polymer n represents a large number ethene poly(ethene) MONOMER POLYMER

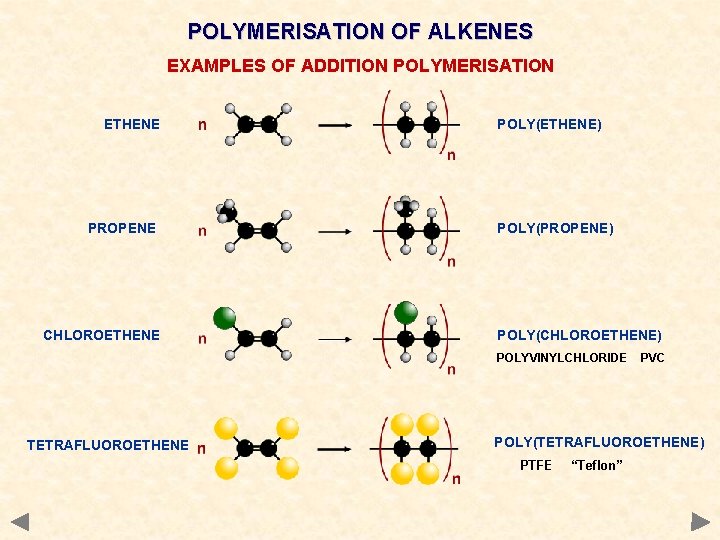
POLYMERISATION OF ALKENES EXAMPLES OF ADDITION POLYMERISATION ETHENE PROPENE CHLOROETHENE POLY(ETHENE) POLY(PROPENE) POLY(CHLOROETHENE) POLYVINYLCHLORIDE TETRAFLUOROETHENE PVC POLY(TETRAFLUOROETHENE) PTFE “Teflon”

POLYMERISATION OF PROPENE - ANIMATION AN EXAMPLE OF ADDITION POLYMERISATION PROPENE MOLECULES DO NOT ALWAYS ADD IN A REGULAR WAY THERE ARE THREE BASIC MODES OF ADDITION ISOTACTIC SYNDIOTACTIC Animation may not work in earlier versions of Powerpoint

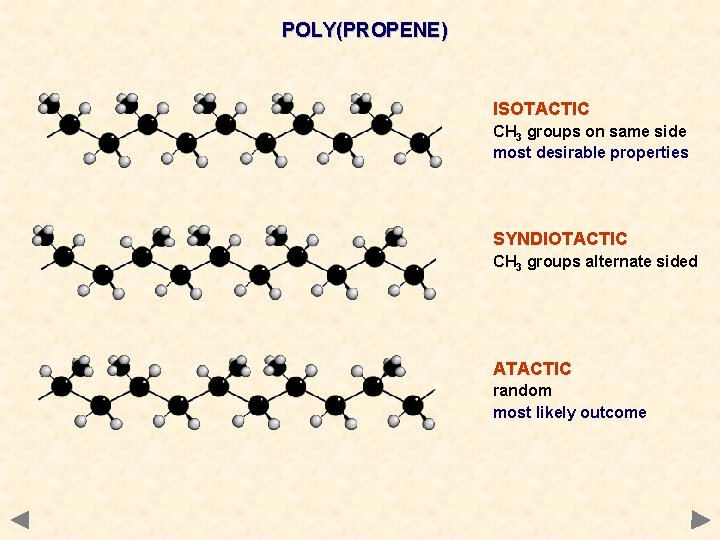
POLY(PROPENE) ISOTACTIC CH 3 groups on same side most desirable properties SYNDIOTACTIC CH 3 groups alternate sided ATACTIC random most likely outcome

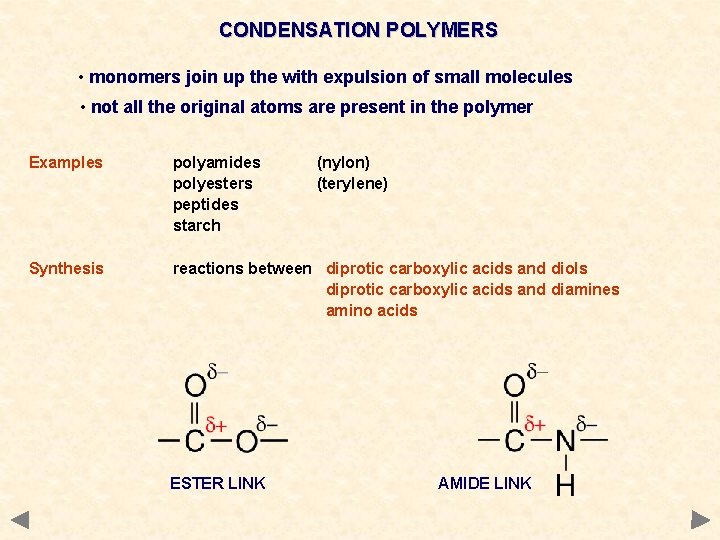
CONDENSATION POLYMERS • monomers join up the with expulsion of small molecules • not all the original atoms are present in the polymer Examples polyamides polyesters peptides starch Synthesis reactions between diprotic carboxylic acids and diols diprotic carboxylic acids and diamines amino acids ESTER LINK (nylon) (terylene) AMIDE LINK

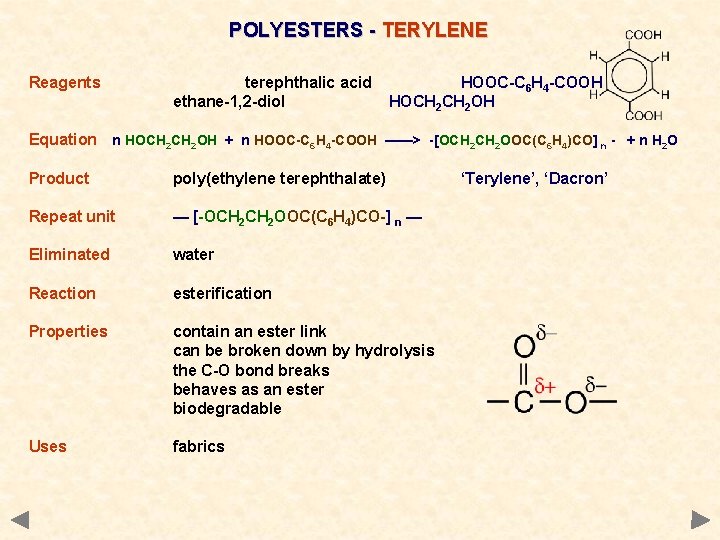
POLYESTERS - TERYLENE Reagents terephthalic acid ethane-1, 2 -diol HOOC-C 6 H 4 -COOH HOCH 2 OH Equation n HOCH 2 OH + n HOOC-C 6 H 4 -COOH ——> -[OCH 2 OOC(C 6 H 4)CO] n - + n H 2 O Product poly(ethylene terephthalate) Repeat unit — [-OCH 2 OOC(C 6 H 4)CO-] n — Eliminated water Reaction esterification Properties contain an ester link can be broken down by hydrolysis the C-O bond breaks behaves as an ester biodegradable Uses fabrics ‘Terylene’, ‘Dacron’

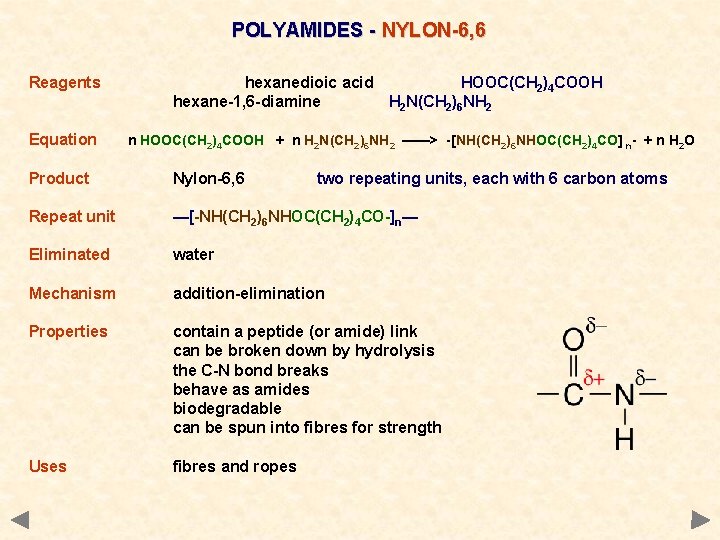
POLYAMIDES - NYLON-6, 6 Reagents Equation hexanedioic acid HOOC(CH 2)4 COOH hexane-1, 6 -diamine H 2 N(CH 2)6 NH 2 n HOOC(CH 2)4 COOH + n H 2 N(CH 2)6 NH 2 ——> -[NH(CH 2)6 NHOC(CH 2)4 CO] n- + n H 2 O Product Nylon-6, 6 two repeating units, each with 6 carbon atoms Repeat unit —[-NH(CH 2)6 NHOC(CH 2)4 CO-]n— Eliminated water Mechanism addition-elimination Properties contain a peptide (or amide) link can be broken down by hydrolysis the C-N bond breaks behave as amides biodegradable can be spun into fibres for strength Uses fibres and ropes

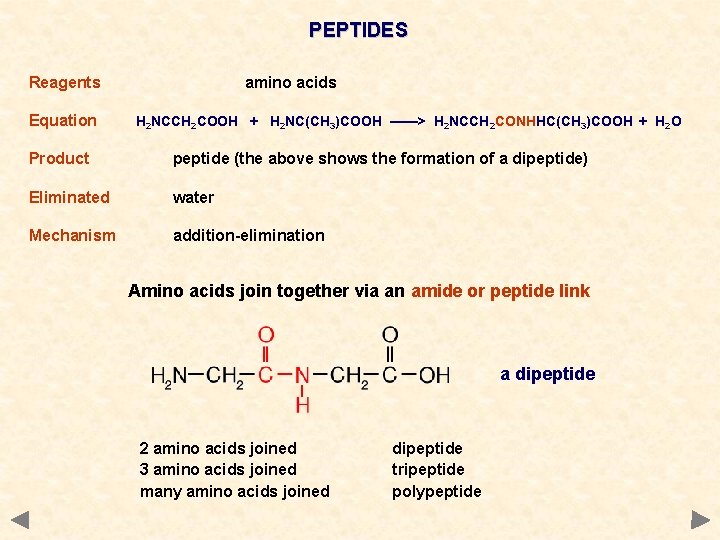
PEPTIDES Reagents Equation amino acids H 2 NCCH 2 COOH + H 2 NC(CH 3)COOH ——> H 2 NCCH 2 CONHHC(CH 3)COOH + H 2 O Product peptide (the above shows the formation of a dipeptide) Eliminated water Mechanism addition-elimination Amino acids join together via an amide or peptide link a dipeptide 2 amino acids joined 3 amino acids joined many amino acids joined dipeptide tripeptide polypeptide

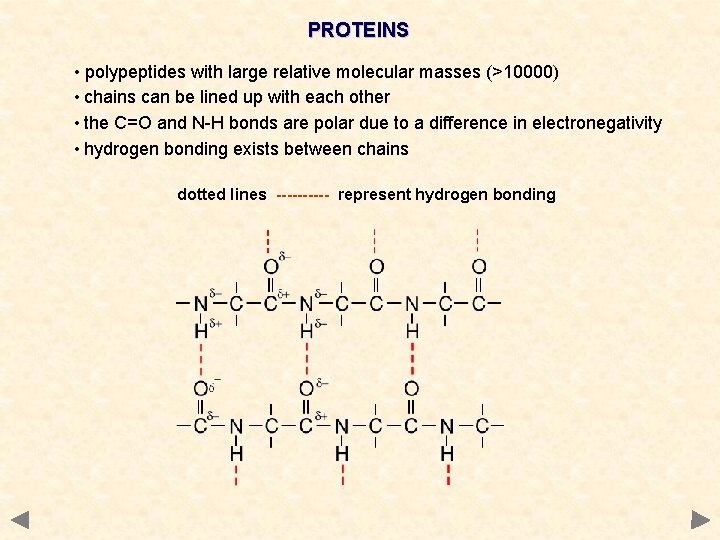
PROTEINS • polypeptides with large relative molecular masses (>10000) • chains can be lined up with each other • the C=O and N-H bonds are polar due to a difference in electronegativity • hydrogen bonding exists between chains dotted lines ----- represent hydrogen bonding