Polymerisation Addition Polymerisation Condensation Polymerisation Uses of polymers







- Slides: 20

Polymerisation Addition Polymerisation Condensation Polymerisation Uses of polymers

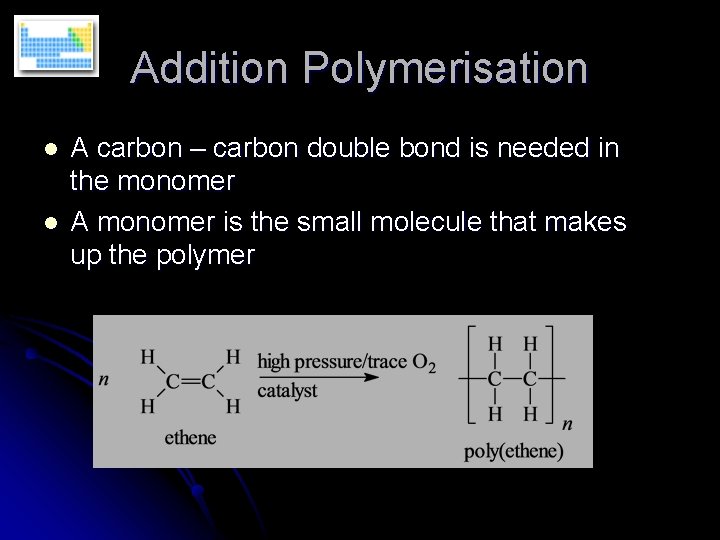
Addition Polymerisation l l A carbon – carbon double bond is needed in the monomer A monomer is the small molecule that makes up the polymer

Addition Polymerisation The polymer is the only product l Involves the opening out of a double bond l The conditions of the reaction can alter the properties of the polymer l Reaction proceeds by a free radical mechanism l Oxygen often used as the initiator l

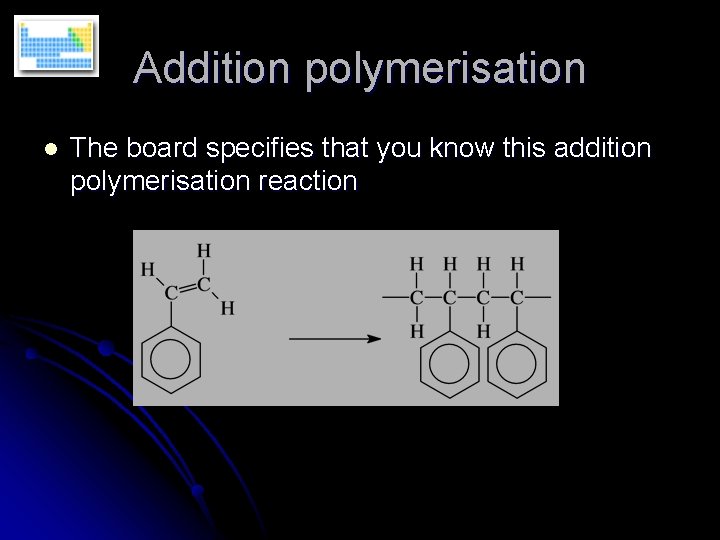
Addition polymerisation l The board specifies that you know this addition polymerisation reaction

Addition polymerisation Conditions are high pressure and an oxygen initiator (to provide the initial free radical). l Monomer = phenylethene l Polymer = poly(phenylethene) l

Addition Polymerisation You are expected to be able to do the following things with addition polymers: l Predict the repeating unit of the polymer given the monomer l Predict the monomer from the polymer – displayed formula and even empirical formula. l Know about stereochemistry of addition polymers. l

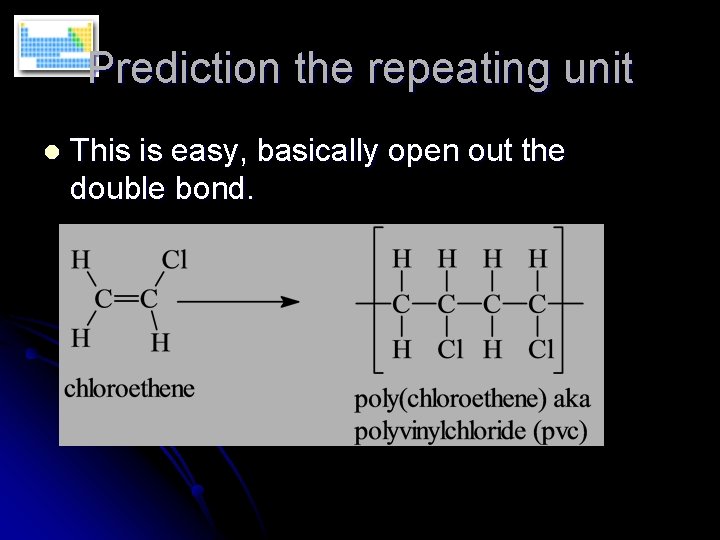
Prediction the repeating unit l This is easy, basically open out the double bond.

Predicting the monomer from the polymer This is kind of the opposite to what you have just done. l They may ask you to draw different formulae. l You need to make sure you can convert repeating units into monomers, and draw a ring around the repeating unit l

Stereoisomerism in Addition polymers. Ziegla and Natta in the 1950 s cam up with a way of controlling the repeating unit. l They won a Joint Nobel prize for their work l The polymerisation process can be controlled used a tin/aluminium catalyst at 50°C and 1. 5 atm l

Stereoisomerism in Addition polymers. Previous to this only one type of poly(ethene) could be made, called LDPE or low density poly(ethane). l The chains formed a tangled mass. l HDPE could now be produced. l This has a much stiffer structure due to areas of crytallinity where the polymer chains are much more ordered. l

Stereoisomerism in Addition polymers. HDPE has a much higher boiling point due to these more ordered regions. l Generally used to make plastic bottles. l Ziegler and Natta also discovered that they could make stereo regular polymers. Isotactic, syndiotactic and atactic. l

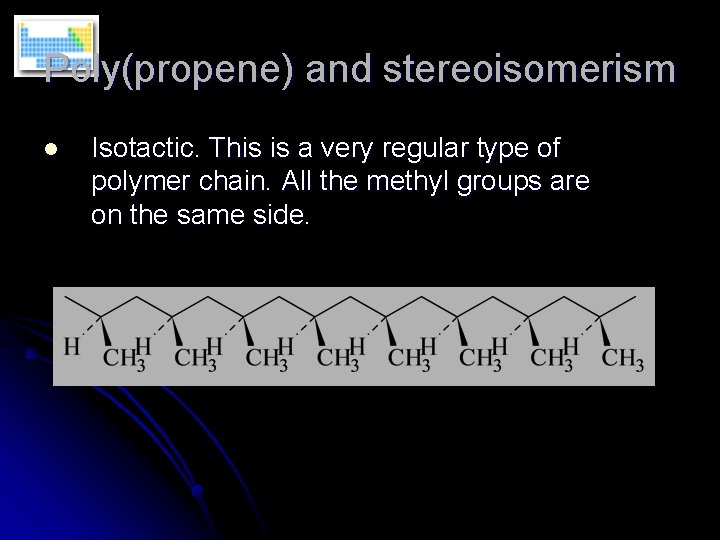
Poly(propene) and stereoisomerism l Isotactic. This is a very regular type of polymer chain. All the methyl groups are on the same side.

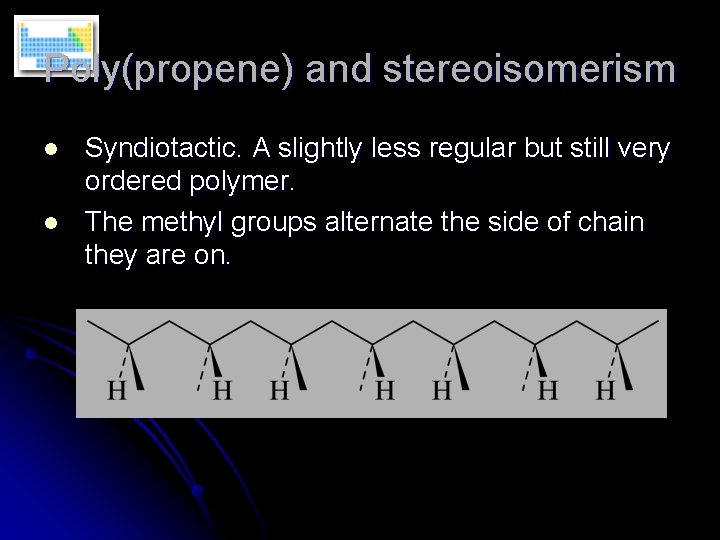
Poly(propene) and stereoisomerism l l Syndiotactic. A slightly less regular but still very ordered polymer. The methyl groups alternate the side of chain they are on.

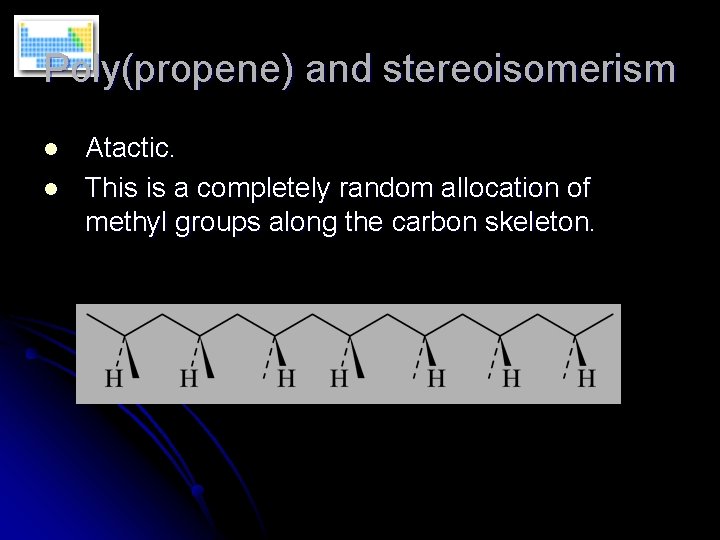
Poly(propene) and stereoisomerism l l Atactic. This is a completely random allocation of methyl groups along the carbon skeleton.

Poly(propene) and stereoisomerism This varying degree of randomness will affect the strength and melting point of the polymer. l The less random, the stronger the polymer and the higher the melting point l This is because in a more ordered polymer they chains can get closer together and hence the van der Waal’s forces will be greater. l

Condensation Polymers l l l Involves 2 monomers that have different functional groups. They also involve the elimination of water or another small molecule. Hence the term condensation polymer. Monomer A + Monomer B Polymer + small molecule (normally water). Common condensation polymers include polyesters (the ester linkage) and polyamides (the amide linkage as in proteins).

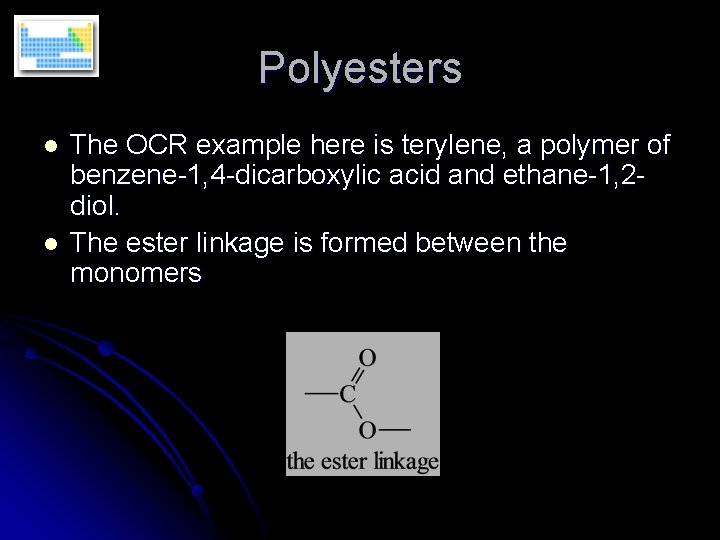
Polyesters l l The OCR example here is terylene, a polymer of benzene-1, 4 -dicarboxylic acid and ethane-1, 2 diol. The ester linkage is formed between the monomers

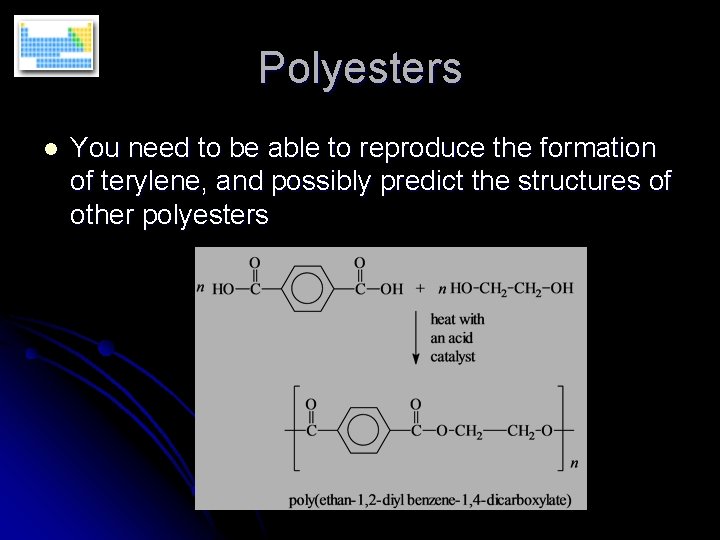
Polyesters l You need to be able to reproduce the formation of terylene, and possibly predict the structures of other polyesters

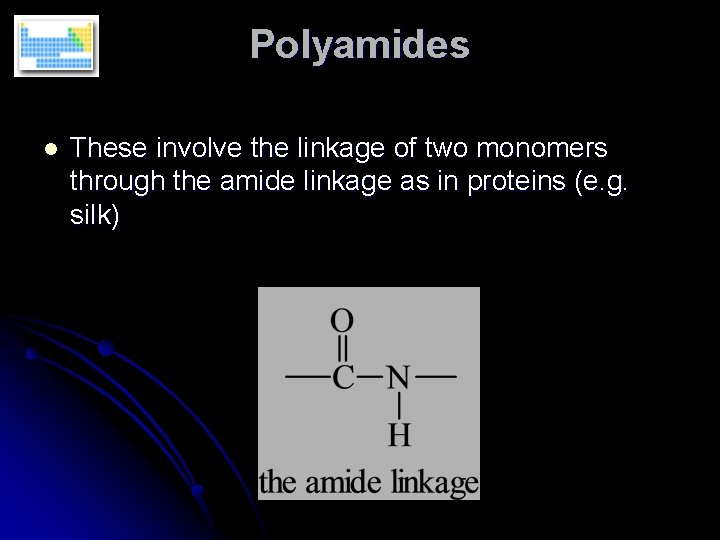
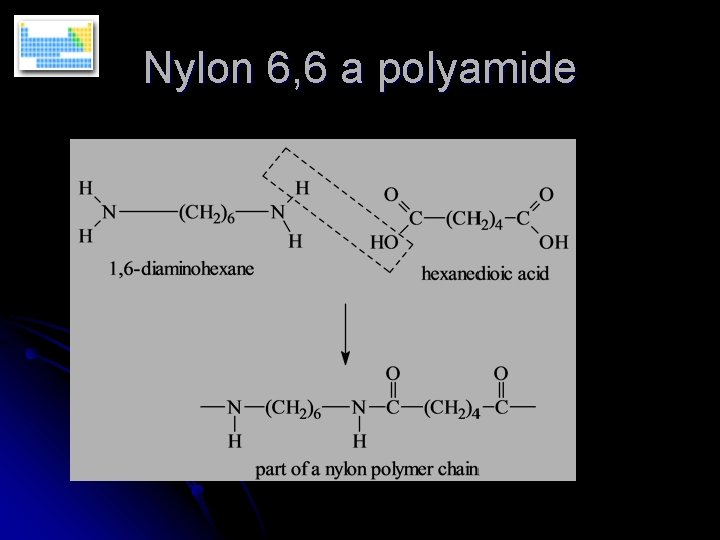
Polyamides l These involve the linkage of two monomers through the amide linkage as in proteins (e. g. silk)

Nylon 6, 6 a polyamide