POLYMERISATION 12 December 2021 C 2 5 Hydrocarbons

POLYMERISATION 12 December 2021 C 2. 5 Hydrocarbons

STARTER - RECAP Draw the mechanism for the reaction of ethane with hydrogen bromide.
USES OF ELECTROPHILIC ADDITION REACTIONS Bromine § Used as a test for an alkene. In the presence of an alkene, bromine decolourises from brown to colourless. Hydrogen § This reaction is called hydrogenation. It is catalysed by transition metals including platinum and palladium but nickel most commonly used. § The reaction hardens oils to make solid edible fats like butter substitutes.

HOW ARE POLYMERS FORMED? Addition polymerisation. The double bonds in alkenes can open up and join together. Many monomer molecules join together to make a large polymer molecule.
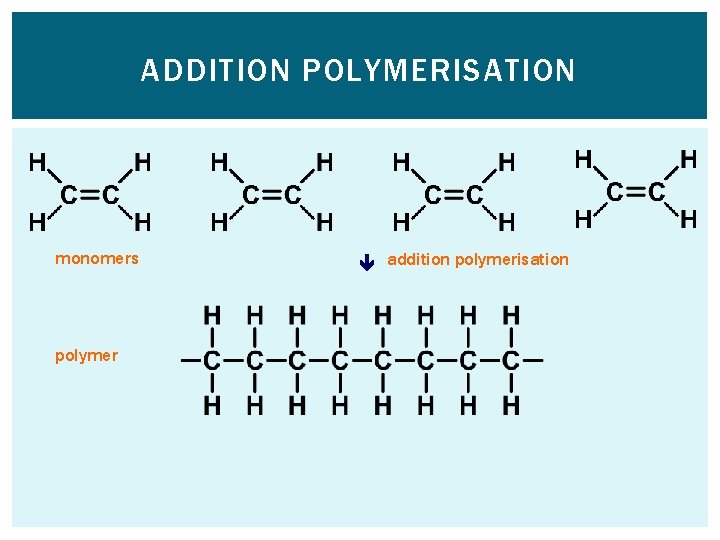
ADDITION POLYMERISATION polymer monomers addition polymerisation

DRAWING POLYMERS Polymers contain thousands of molecules, so how do we draw these easily? A shorthand formula can be used to show the ‘repeating unit’ § The ’n’ means that the polymer contains a very large number of the repeating unit shown in the brackets.
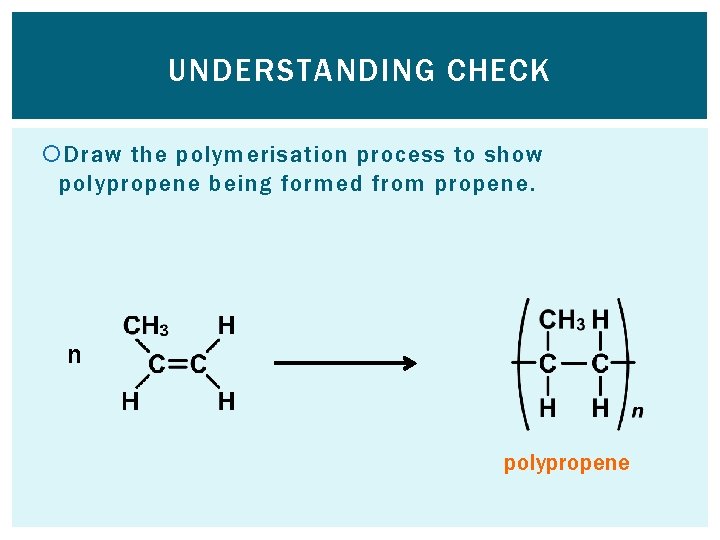
UNDERSTANDING CHECK Draw the polymerisation process to show polypropene being formed from propene. n polypropene
POLYMER PROPERTIES Polymer properties can be changed by changing their structure (branched/straight chains). Changing the temperature or catalyst or pressure at which the polymer is made can change the properties Polymers can also be made from substituted alkenes (alkenes where H atoms have been substituted for halogens for example).
USES OF POLYMERS Using the internet or textbook, make notes on the following plastics. Ensure you include: § § The monomer The repeating unit Properties Uses Poly(ethene) Poly(propene) Poly(chloroethene) Poly(phenylethene)
- Slides: 9