Polymer Technology Chapter 1 Introduction to Polymer Science


- Slides: 12

Polymer Technology Chapter 1 Introduction to Polymer Science

BASIC DEFINITIONS • The terms thermoplastic, polymer or macromolecule are used to explain a wide range of materials which have one feature in common: they are all large molecules created by chemically linking smaller entities of monomers • The term ‘polymer’ is derived from two greek letters: poly meaning many and mer, which is an abbreviation of the word monomer, which is the primary building block from which the macromolecule or the polymer is created. • The alternative name for a polymer is a macromolecule, which indicates that it is a high molar mass species. • The term macromolecule or thermoplastic does not necessarily mean that all the elements along the backbone of the molecule are the same. • The term thermoplastic has come to be used to explain a wide range of synthetic macromolecules.

BASIC DEFINITIONS • Polymers or macromolecules coluld be either rigid or flexible and might either be brittle or very elastic. • Unlike metals and ceramics, any change in temperature can convert a brittle rigid thermoplastic into a soft and extensible elastomer, which is kind of macromolecule. • Most of polymeric structures are based on linking molecules that have a carbon– carbon bonded structure. In addition, there are very important materials based on inorganic bonded structures such as silicon– oxygen (R 2 Si 02)n, phosphorus– nitrogen (P–N)n and boron– nitrogen (B–N)n. • The specified materials have certain applications and are beyond the scope of this chapter.

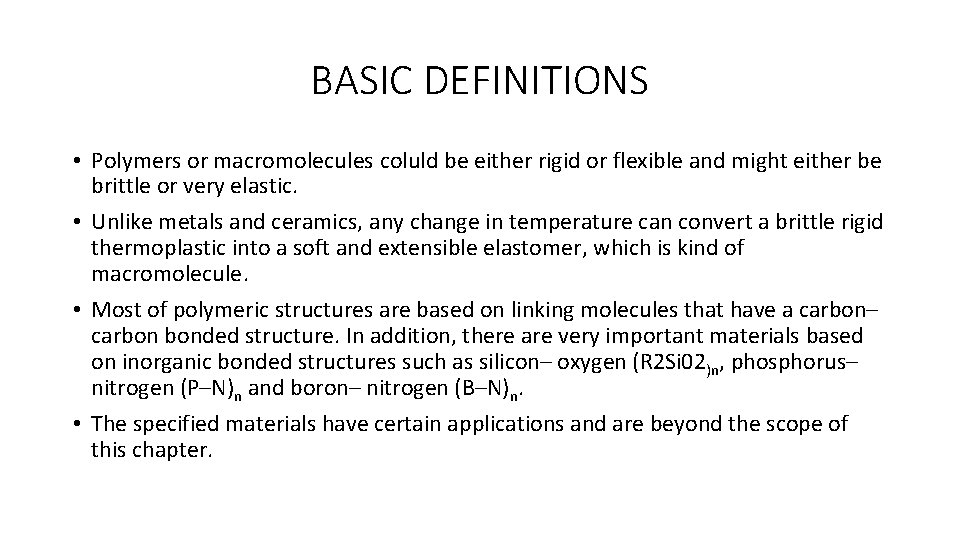
BASIC DEFINITIONS • As an example, the styrene molecule contains a double bond. The styrene chemical structure and its polymer form are illustrated below. • Scientiest studied on polymer chemistry have devised methods of opening this double bond on the styrene molecule so that literally thousands of styrene molecules become linked together in the macromolecular structure. • The resulting structure, illustrated below in square brackets, is the polymer polystyrene. • The single unit ‘Styrene’ itself is referred to as a monomer, which is defined as any molecule, which can be converted to a polymer by combining with other molecules of the same or different type. • The unit in square brackets of polystyrene is called the repeating unit.

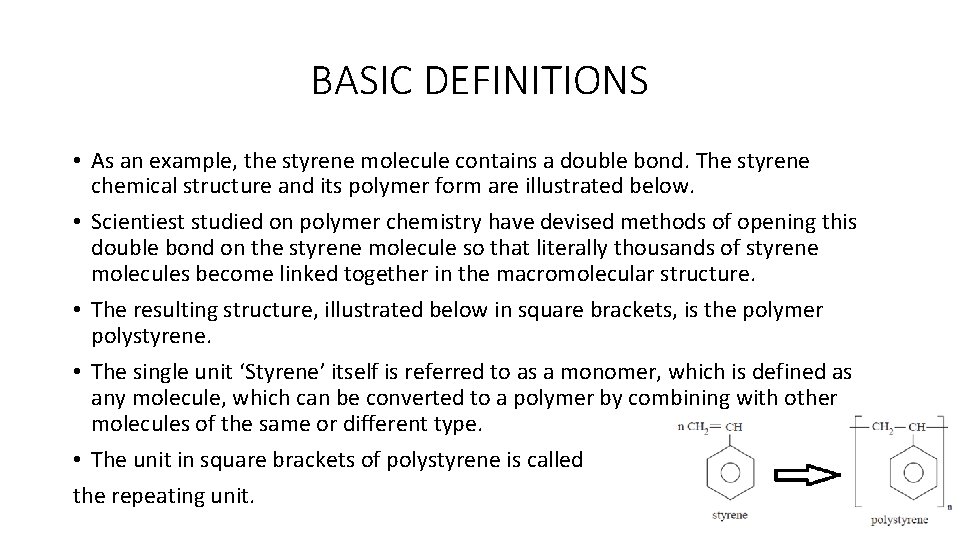
BASIC DEFINITIONS • The conversion of the single unit ‘monomer’ to the macromolecules or polymer includes a rearrangement of electrons on the monomer. • In the case of the specified polymer ‘polystyrene’, the polymer is derived from a single monomer ‘styrene’. • Other examples of polymers of this type are polyethylene, polyacrylonitrile, and polypropylene. The repeating units of the specified macromolecules are given below:

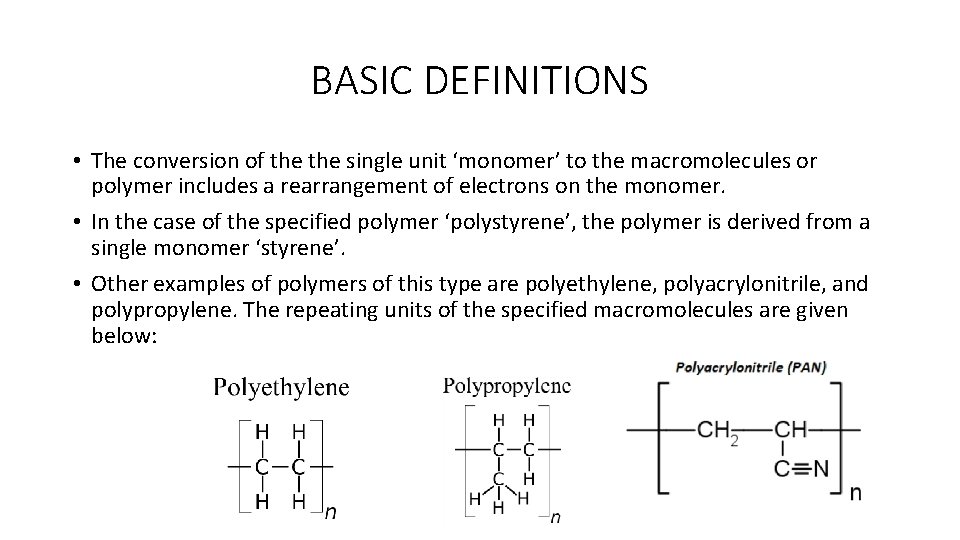
BASIC DEFINITIONS • On the contrary, some polymers can be derived from the mutual reaction of two or more monomers that are chemically similar but not identical. • As an example, poly(hexamethylene adipamide) or nylon 6, 6 can be derived from the reaction of hexamethylenediamine and adipic acid. • The repeating unit in this case consists of two structural units: • The term, n, indicates the number of repeating units bonded together in the polymer chain (molecule). • This term also gives us as the degree of polymerization (DP).

BASIC DEFINITIONS • The degree of polymerization (DP) specifies the length of the macromolecules. • Polymerization reaction takes place by the sequential reactions of monomers, which provide the linking of the repeating units along the chain. • First of all, monomers start to form a dimer, which in turn reacts with another monomer to form a trimer and it goes so on. • By the way, the reaction can takes place between dimers, trimers, or any molecular species within the reaction mixture to form a progressively larger molecule compared to the unit molecule ‘monomer’. • Hence, a series of linkages is built between the repeating units of the monomers, and the resulting polymer structure is called the polymer chain. • Low-molecular-weight synthesis products of the polymerization reaction such as dimers, trimers, tetramers, etc. , are callled as oligomers.

BASIC DEFINITIONS • The oligomeras generally possess undesirable thermal and mechanical properties compared the macromolecules because of the size difference. • A high degree of polymerization is desired for a material to get useful thermal and mechanical properties. • For example, polystyrene, with a degree of polymerization of n=7, is a viscous liquid. • On the other hand, the commercial grade polystyrene is a solid with a degree of polymerization of n=1000. • In addition, the degree of polymerization can be used to quantify the molecular length or size of a polymer. MW(Polymer) = DP × MW(Repeat Unit). • As an example, polystyrene have eight carbon atoms and eight hydrogen atoms in the repeating unit. Thus, the molecular weight of the repeating unit is equal to 104.

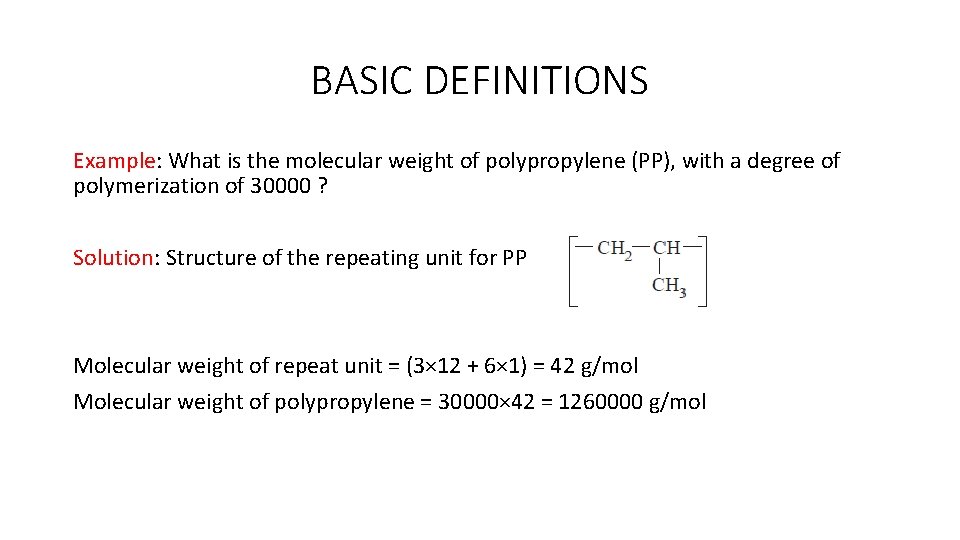
BASIC DEFINITIONS Example: What is the molecular weight of polypropylene (PP), with a degree of polymerization of 30000 ? Solution: Structure of the repeating unit for PP Molecular weight of repeat unit = (3× 12 + 6× 1) = 42 g/mol Molecular weight of polypropylene = 30000× 42 = 1260000 g/mol

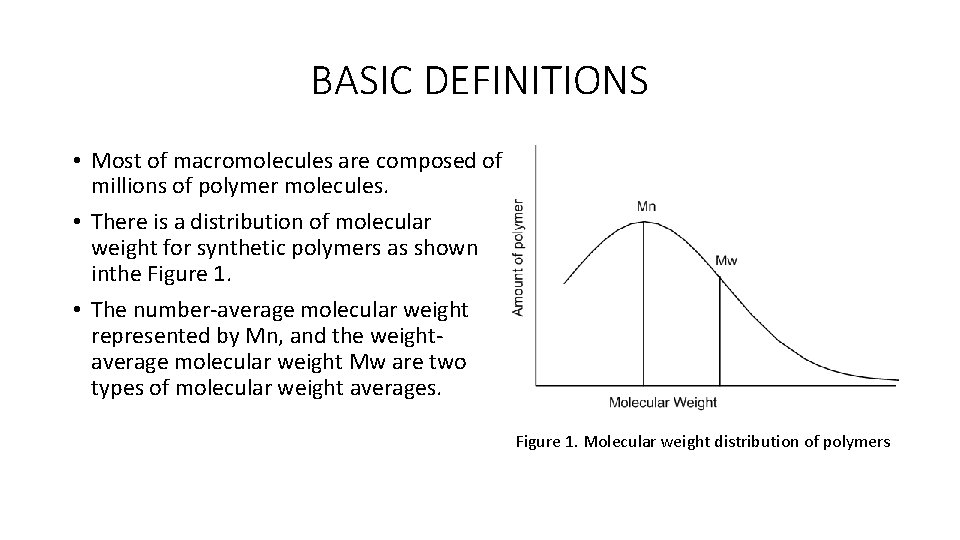
BASIC DEFINITIONS • Most of macromolecules are composed of millions of polymer molecules. • There is a distribution of molecular weight for synthetic polymers as shown inthe Figure 1. • The number-average molecular weight represented by Mn, and the weightaverage molecular weight Mw are two types of molecular weight averages. Figure 1. Molecular weight distribution of polymers

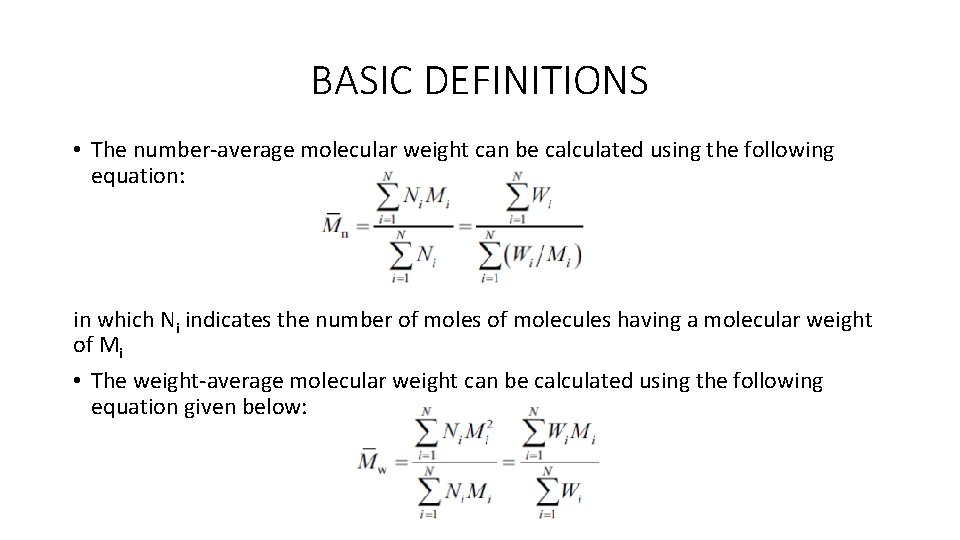
BASIC DEFINITIONS • The number-average molecular weight can be calculated using the following equation: in which Ni indicates the number of moles of molecules having a molecular weight of Mi • The weight-average molecular weight can be calculated using the following equation given below:

References • Robert O. Ebewele, «POLYMER SCIENCE AND TECHNOLOGY» , CRC Press, 2000. • Fried, Joel R. , «Polymer science and technology» , Prentice Hall, Third edition.