POLYMER CRYSTALLINITY The amorphous nature of polymers is



- Slides: 18

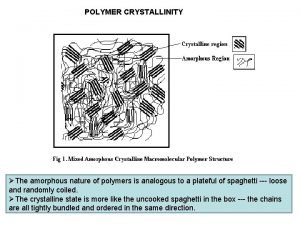
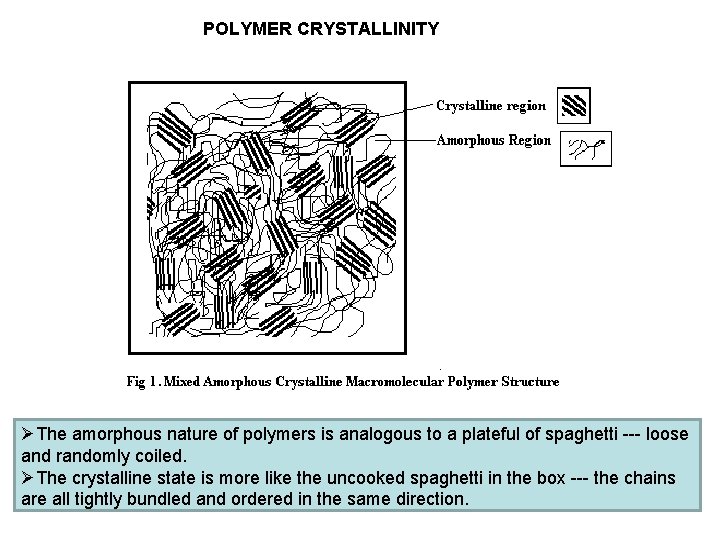
POLYMER CRYSTALLINITY ØThe amorphous nature of polymers is analogous to a plateful of spaghetti --- loose and randomly coiled. ØThe crystalline state is more like the uncooked spaghetti in the box --- the chains are all tightly bundled and ordered in the same direction.

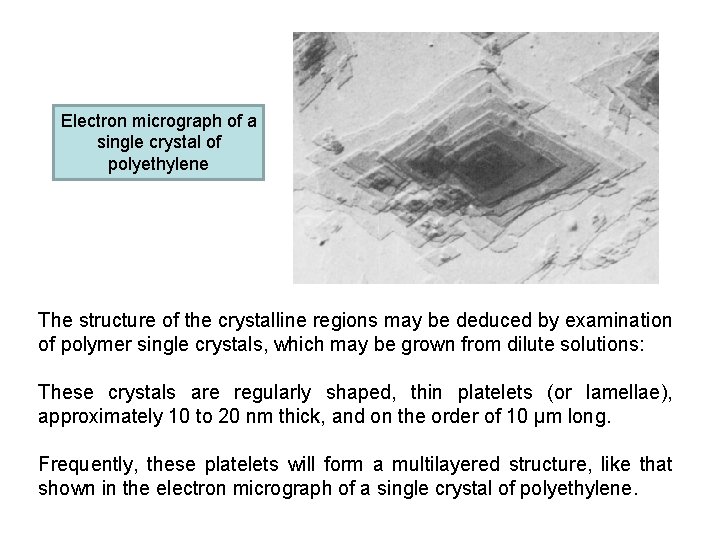
Electron micrograph of a single crystal of polyethylene The structure of the crystalline regions may be deduced by examination of polymer single crystals, which may be grown from dilute solutions: These crystals are regularly shaped, thin platelets (or lamellae), approximately 10 to 20 nm thick, and on the order of 10 μm long. Frequently, these platelets will form a multilayered structure, like that shown in the electron micrograph of a single crystal of polyethylene.

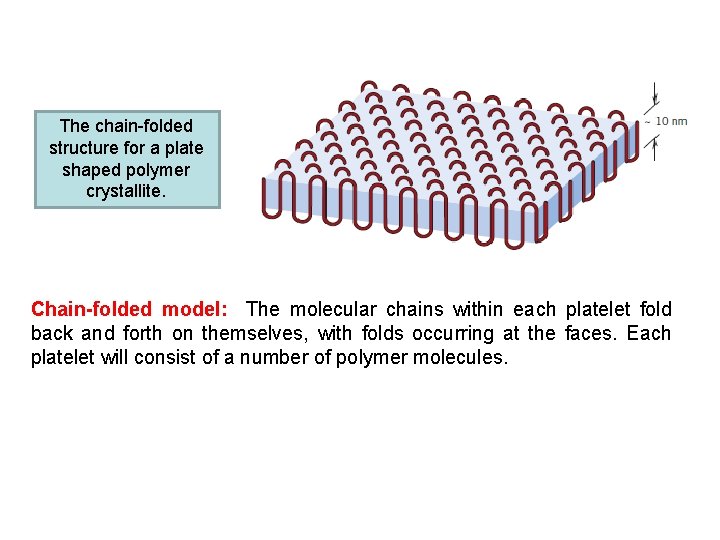
The chain-folded structure for a plate shaped polymer crystallite. Chain-folded model: The molecular chains within each platelet fold back and forth on themselves, with folds occurring at the faces. Each platelet will consist of a number of polymer molecules.

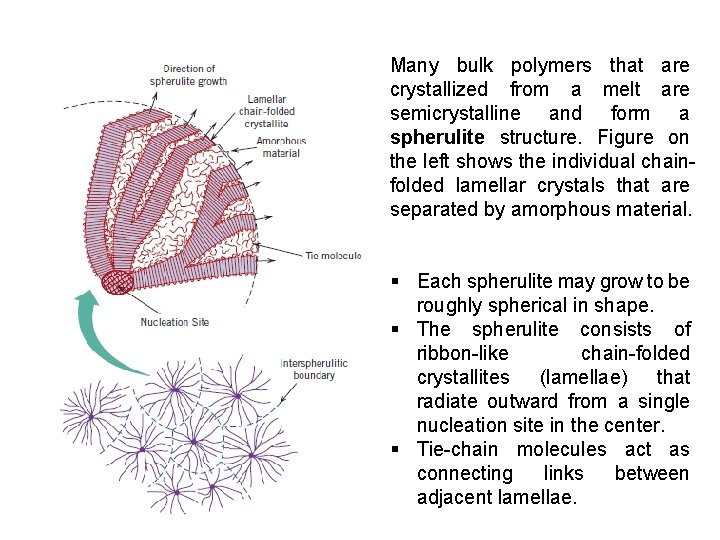
Many bulk polymers that are crystallized from a melt are semicrystalline and form a spherulite structure. Figure on the left shows the individual chainfolded lamellar crystals that are separated by amorphous material. § Each spherulite may grow to be roughly spherical in shape. § The spherulite consists of ribbon-like chain-folded crystallites (lamellae) that radiate outward from a single nucleation site in the center. § Tie-chain molecules act as connecting links between adjacent lamellae.

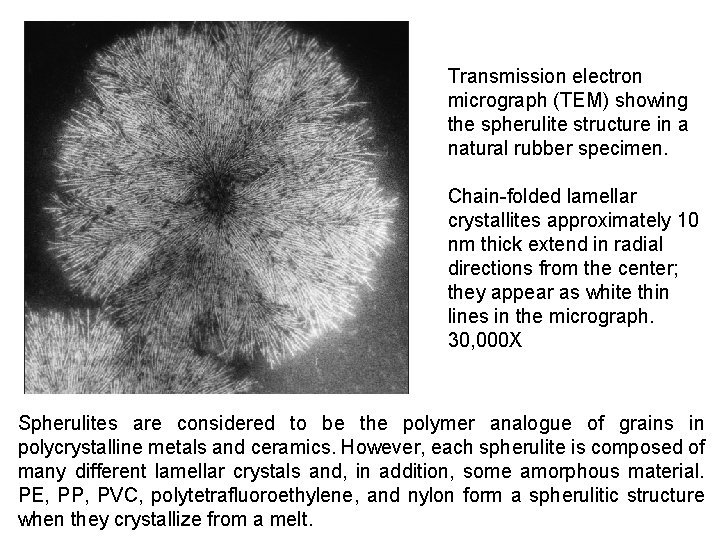
Transmission electron micrograph (TEM) showing the spherulite structure in a natural rubber specimen. Chain-folded lamellar crystallites approximately 10 nm thick extend in radial directions from the center; they appear as white thin lines in the micrograph. 30, 000 X Spherulites are considered to be the polymer analogue of grains in polycrystalline metals and ceramics. However, each spherulite is composed of many different lamellar crystals and, in addition, some amorphous material. PE, PP, PVC, polytetrafluoroethylene, and nylon form a spherulitic structure when they crystallize from a melt.

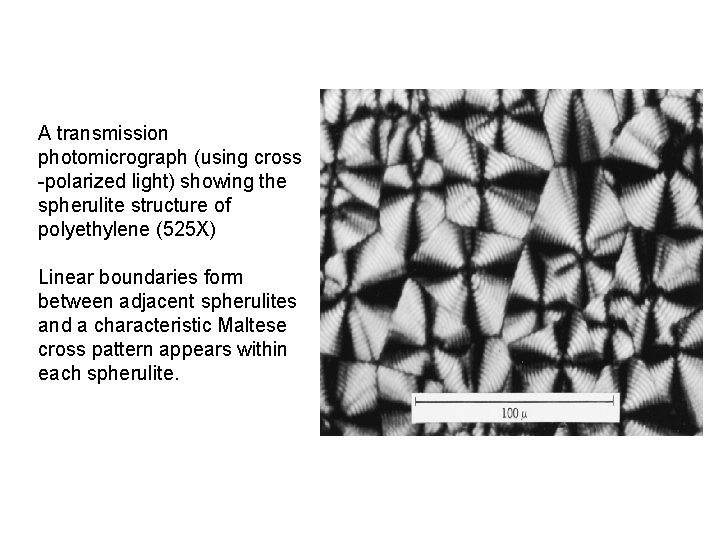
A transmission photomicrograph (using cross -polarized light) showing the spherulite structure of polyethylene (525 X) Linear boundaries form between adjacent spherulites and a characteristic Maltese cross pattern appears within each spherulite.

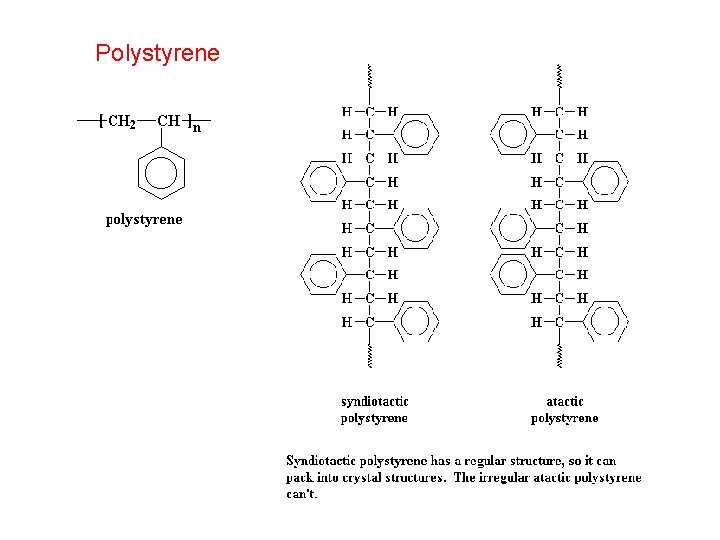
Polystyrene

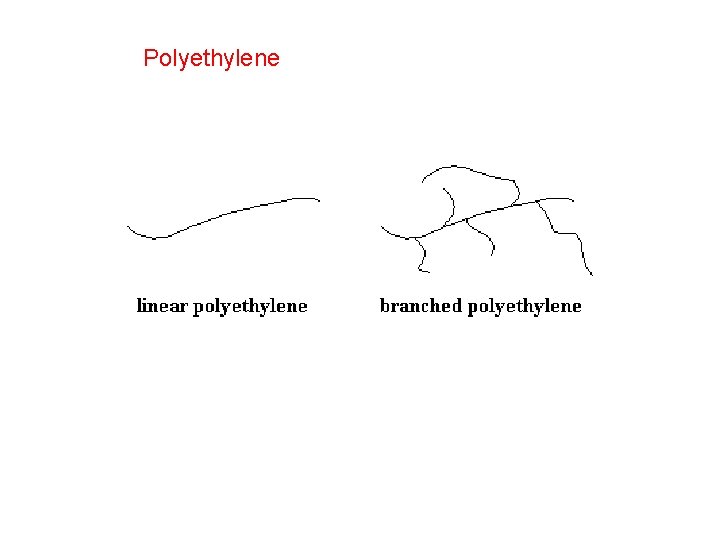
Polyethylene

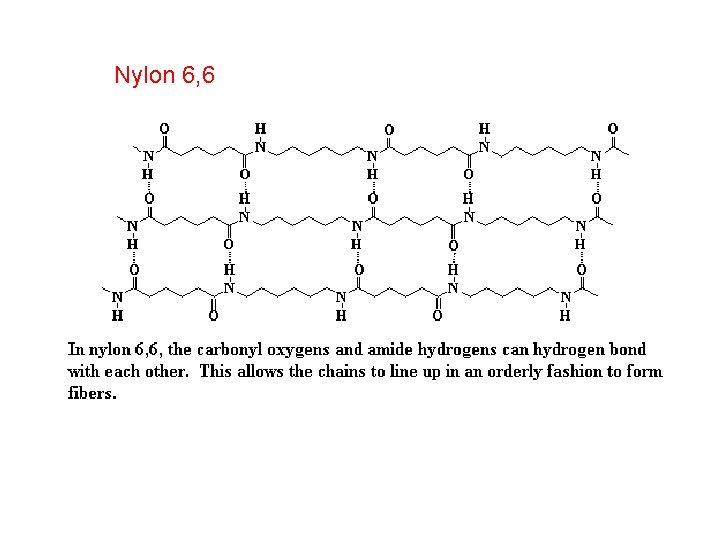
Nylon 6, 6

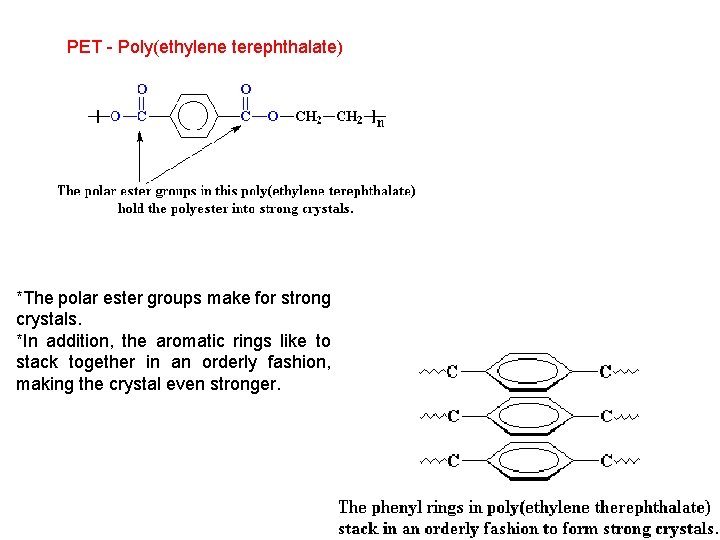
PET - Poly(ethylene terephthalate) *The polar ester groups make for strong crystals. *In addition, the aromatic rings like to stack together in an orderly fashion, making the crystal even stronger.

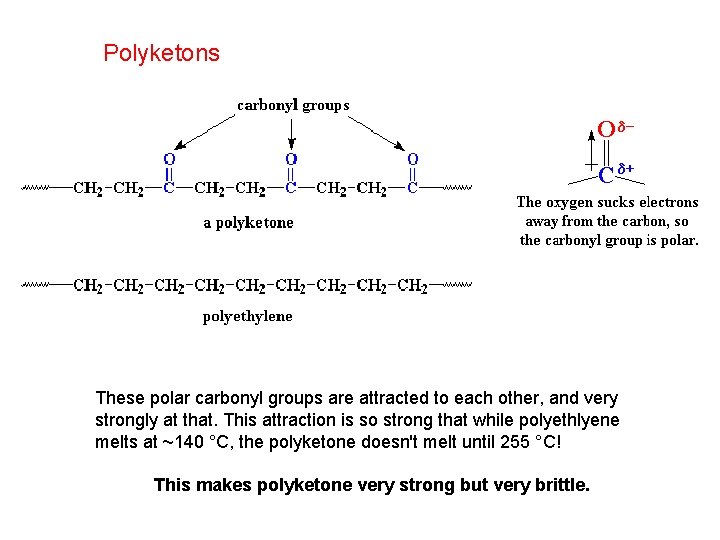
Polyketons These polar carbonyl groups are attracted to each other, and very strongly at that. This attraction is so strong that while polyethlyene melts at ~140 °C, the polyketone doesn't melt until 255 °C! This makes polyketone very strong but very brittle.

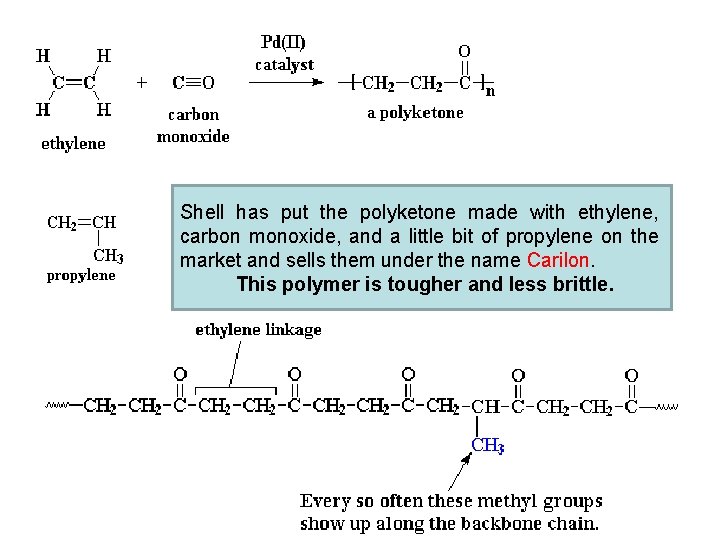
Shell has put the polyketone made with ethylene, carbon monoxide, and a little bit of propylene on the market and sells them under the name Carilon. This polymer is tougher and less brittle.

Carilon Thermoplastic Polymers

SRI (nonprofit research institute) International offers Carilon thermoplastic polymers for multiple applications in the engineering thermoplastic and fiber markets. Originally developed by Shell Oil Company and now available for license exclusively through SRI, Carilon polymers offer superior strength, wear and low permeability. These features make them ideal for use in automotive parts; electrical and electronics systems; business machines and consumer appliances; film, fiber and protective coatings; laboratory supplies, and industrial applications. Representing the next wave in high-performance polymeric materials, Carilon plastics are based on a semicrystalline thermoplastic technology, exhibiting performance characteristics that are maintained even at high temperatures. Carilon polymers offer a broad range of features: • Outstanding chemical resistance and low permeability • Superior strength, wear and friction characteristics • High resistance to fatigue, creep, swelling and repetitive deformation • Excellent balance of stiffness and toughness over a wide temperature range • High-quality moldings at short cycle times • Resistance to a variety of fuels, organic solvents and aggressive aqueous media • Flame retardancy

Article: Shell Abandons Carilon, Sells Polyurethanes (Company Business and Marketing)(Brief Article) Article from: Chemical Week Article date: February 23, 2000

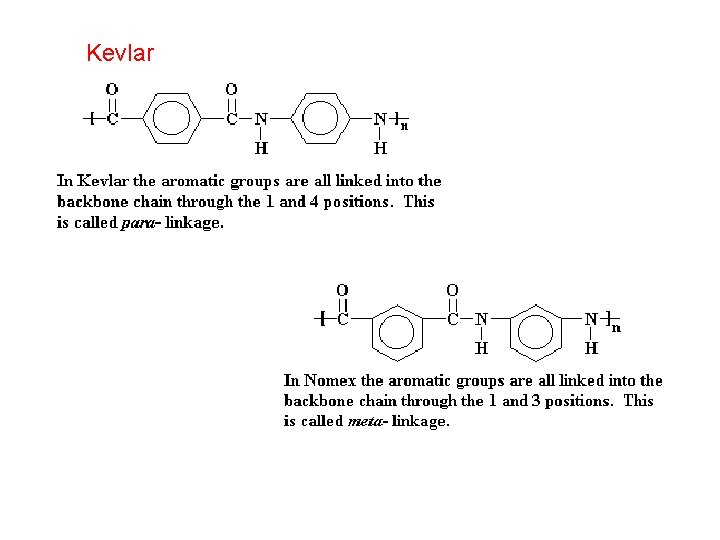
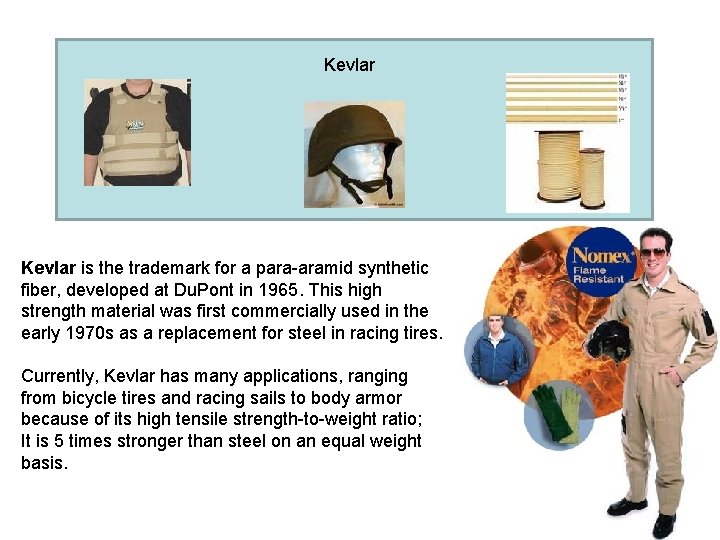
Kevlar

Kevlar is the trademark for a para-aramid synthetic fiber, developed at Du. Pont in 1965. This high strength material was first commercially used in the early 1970 s as a replacement for steel in racing tires. Currently, Kevlar has many applications, ranging from bicycle tires and racing sails to body armor because of its high tensile strength-to-weight ratio; It is 5 times stronger than steel on an equal weight basis.

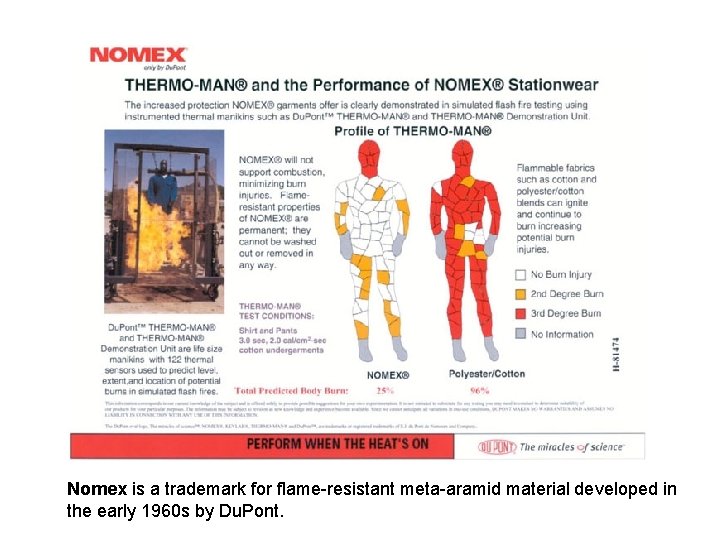
Nomex is a trademark for flame-resistant meta-aramid material developed in the early 1960 s by Du. Pont.
 Fringed micelle model
Fringed micelle model Crystallinity
Crystallinity Alkaptouria
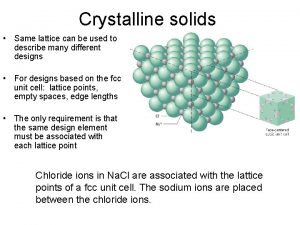
Alkaptouria Example of crystalline solid
Example of crystalline solid The amorphous body of the amoeba has no discernable
The amorphous body of the amoeba has no discernable Microscopic analysis of urine
Microscopic analysis of urine Mit
Mit Crystal solid and amorphous solid
Crystal solid and amorphous solid Fauvism pronunciation
Fauvism pronunciation Honors its atomic
Honors its atomic Centrifuged
Centrifuged Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Amorphous disk mark
Amorphous disk mark Characteristics of solid state
Characteristics of solid state Law of constancy of interfacial angle
Law of constancy of interfacial angle Nature and nature's law lay hid in night meaning
Nature and nature's law lay hid in night meaning Determinace lidské psychiky
Determinace lidské psychiky Merester
Merester Homochain polymers
Homochain polymers