Polymer Chemistry Polymer Characteristics and Classifications Ms Mandel











- Slides: 34

Polymer Chemistry Polymer Characteristics and Classifications Ms. Mandel Honors Physical Science

D 15 O Explain the general formation and structure of carbon-based polymers, including synthetic polymers, such as polyethylene, and biopolymers, such as carbohydrate.

D 16 O Explain how simple chemical monomers can be combined to create linear, branched and/or cross-linked polymers.

D 17 O Explain how the chemical structure of polymers affects their physical properties.

Learning Targets O I can explain how the structure of a polymer affects its properties. O I can differentiate between thermoset and thermoplastic polymers. O I can explain how polymers are synthesized.

Polymers O What is a Monomer? O The individual units that join together to form a polymer. O What is a Polymer? O A very long molecule composed of repeating units connected by covalent bonds.

Characterizing a Polymer O Structure O Classification O Synthesis

Structure of a Polymer O Skeletal Structure O Chemical Structure

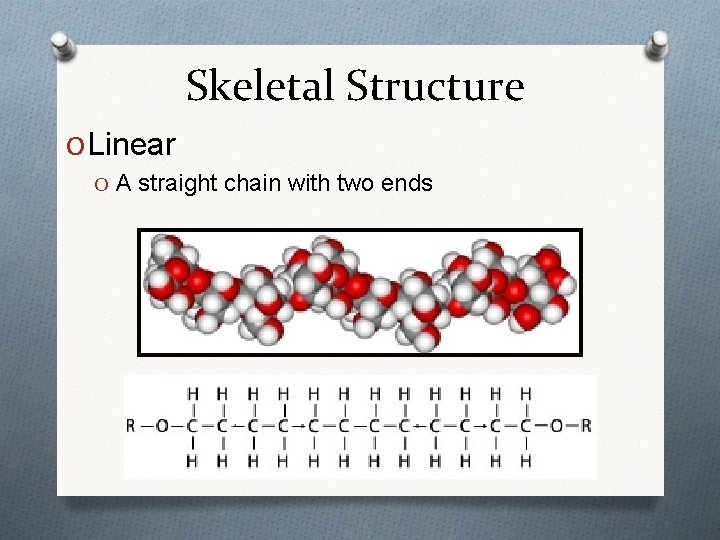
Skeletal Structure O Linear O A straight chain with two ends

Linear Structure O What properties might a linear structure produce? O Short and long chains O Long molecules can get tangled up in each other and stick together better O Long molecules are harder and have higher melting points O Short chains pass over each other easily and are softer and “squishy” O No side chains means it has a higher density because more molecules can pack into the same amount of space O The molecules can slide over each other more easily if there is nothing in the way to stop them O Stretchy

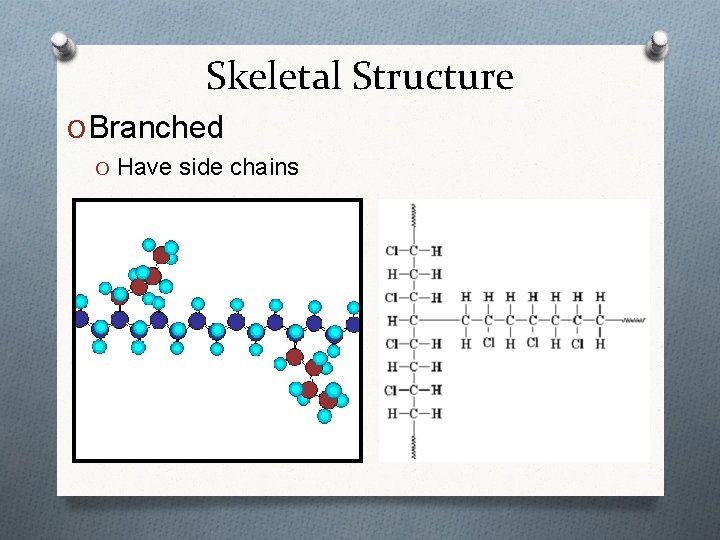
Skeletal Structure O Branched O Have side chains

Branched Structure O What properties might a branched structure produce? O If chains are branched then they cannot pack close together = low density O Branches catch on each other, preventing chains from sliding past one other O Plastic is less stretchy

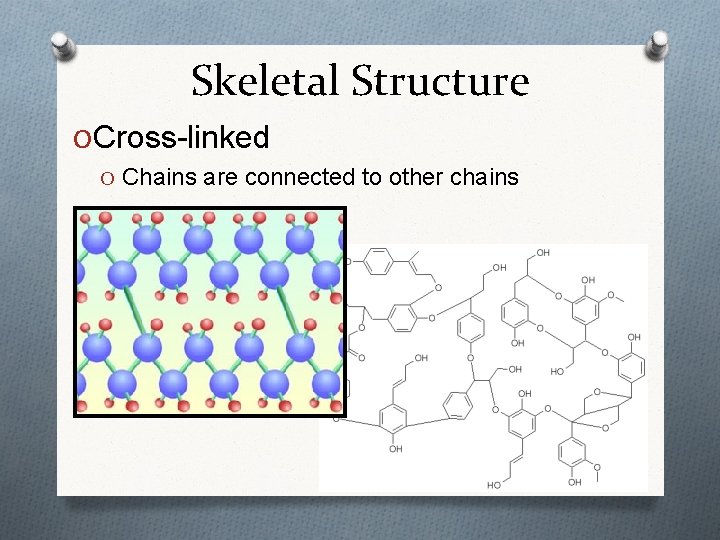
Skeletal Structure O Cross-linked O Chains are connected to other chains

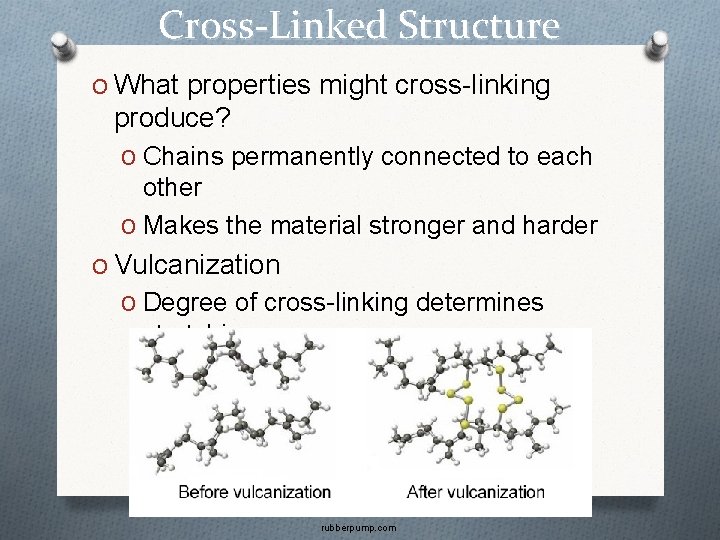
Cross-Linked Structure O What properties might cross-linking produce? O Chains permanently connected to each other O Makes the material stronger and harder O Vulcanization O Degree of cross-linking determines stretchiness rubberpump. com

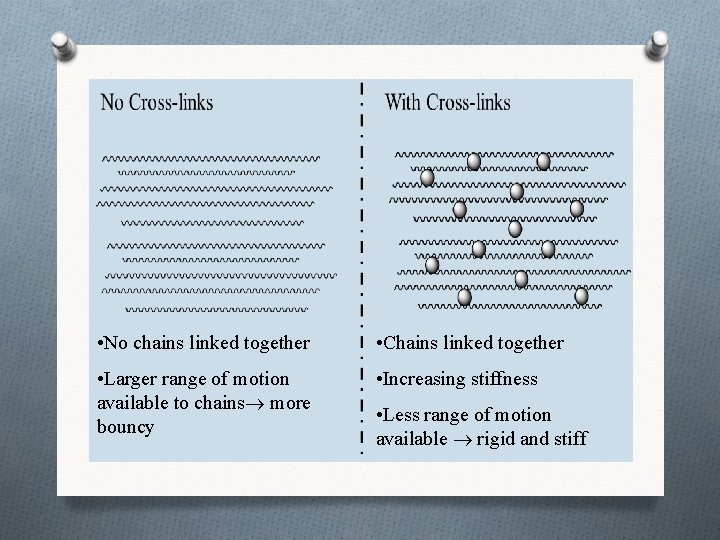
• No chains linked together • Chains linked together • Larger range of motion available to chains more bouncy • Increasing stiffness • Less range of motion available rigid and stiff

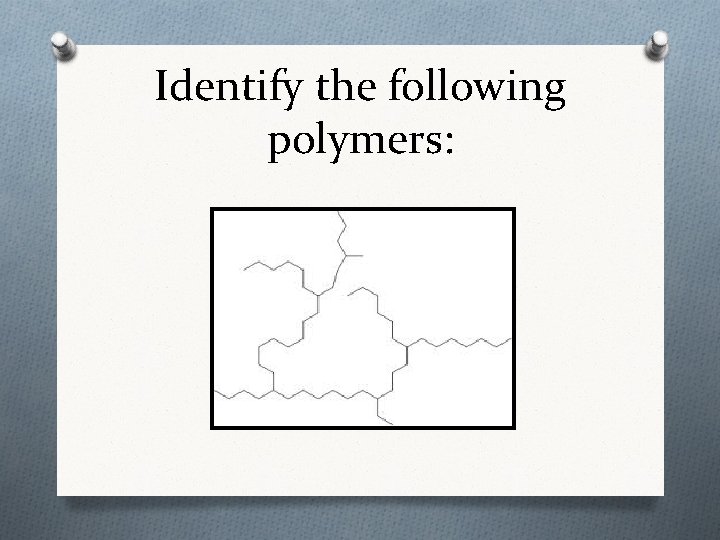
Identify the following polymers:


Learning Target Checkpoint O How does structure determine the property of a polymer?

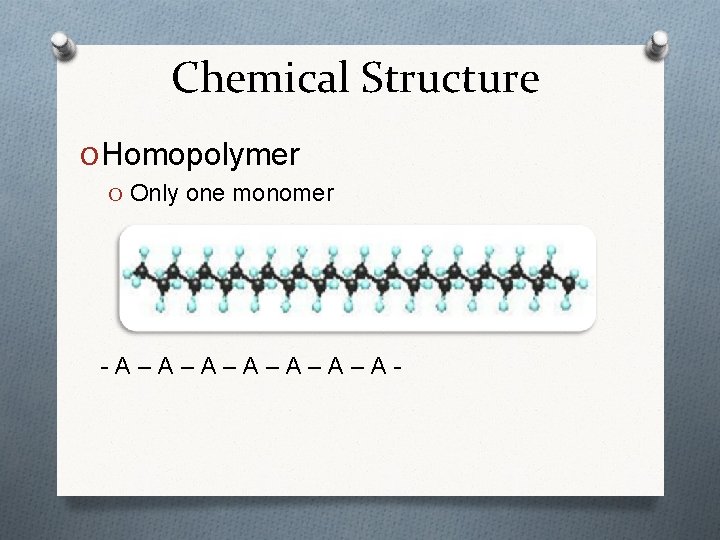
Chemical Structure O Homopolymer O Only one monomer -A–A–A–A-

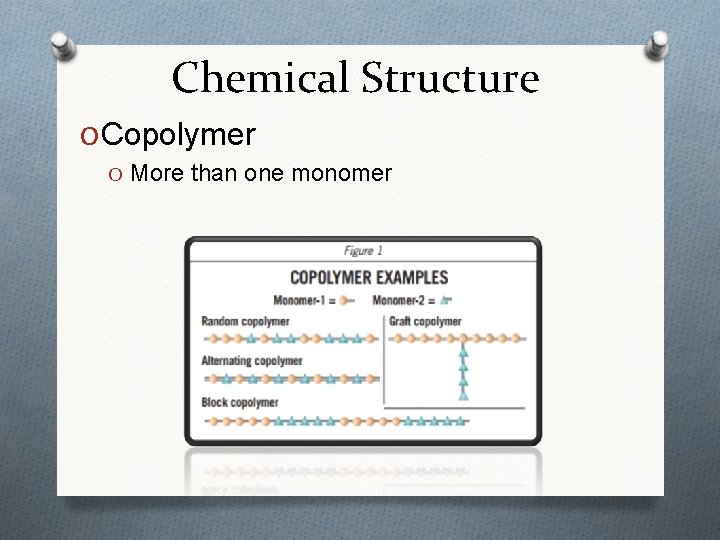
Chemical Structure O Copolymer O More than one monomer

Classifications O Thermoplastic O Thermoset O Elastomer

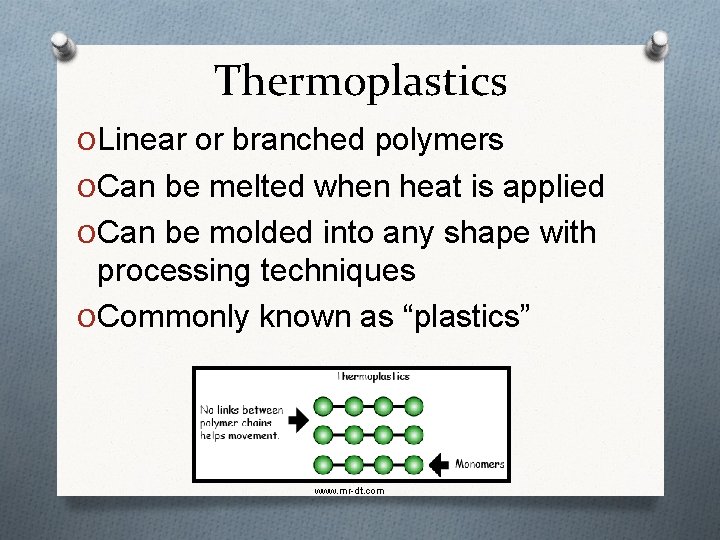
Thermoplastics O Linear or branched polymers O Can be melted when heat is applied O Can be molded into any shape with processing techniques O Commonly known as “plastics” www. mr-dt. com

Thermoplastics O Examples: bottles, grocery bags, water piping, rope, fishing line, car parts, etc. O Most are recyclable www. petervaldivia. com

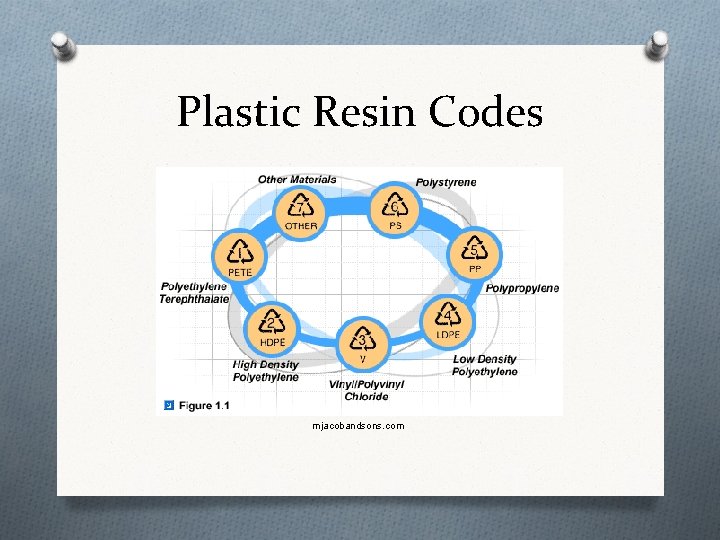
Plastic Resin Codes mjacobandsons. com

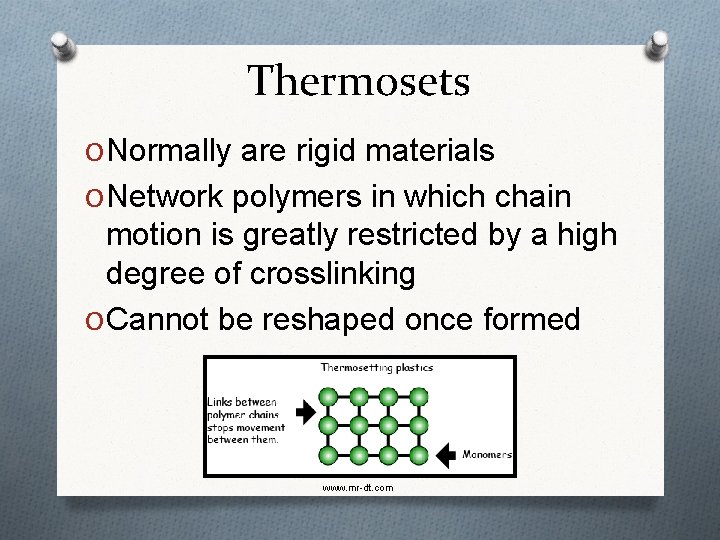
Thermosets O Normally are rigid materials O Network polymers in which chain motion is greatly restricted by a high degree of crosslinking O Cannot be reshaped once formed www. mr-dt. com

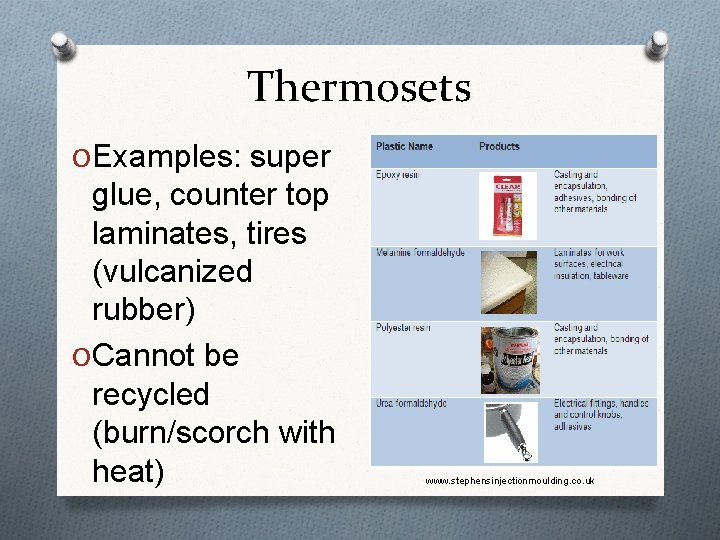
Thermosets O Examples: super glue, counter top laminates, tires (vulcanized rubber) O Cannot be recycled (burn/scorch with heat) www. stephensinjectionmoulding. co. uk

What are Elastomers? O Thermoplastics O Thermosets O Natural O Synthetic O Can be stretched to many times their original length O Can bounce back into their original shape without permanent deformation O Low degree of crosslinking

Elastomers O Uses: examination gloves, rubber bands, bouncing balls, hoses, medical, etc. O Not recyclable www. glstpes. com

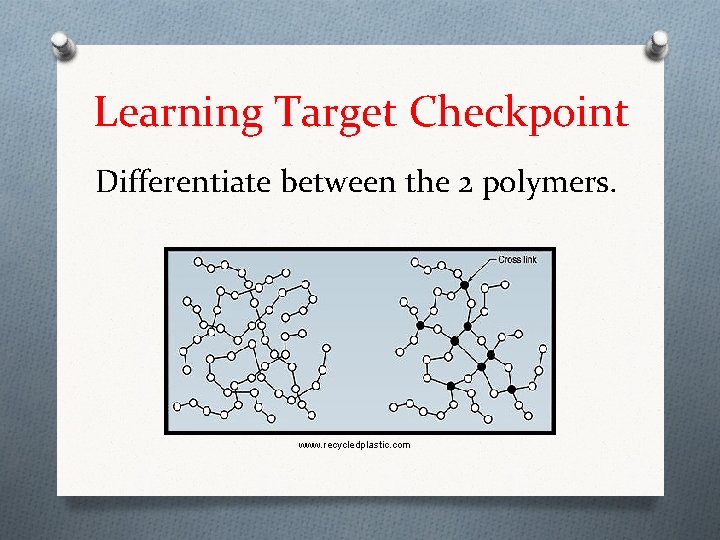
Learning Target Checkpoint Differentiate between the 2 polymers. www. recycledplastic. com

Polymer Chemistry Polymer Synthesis

Addition Polymerization (Polyaddition) O Reactions in which monomers combine without the formation of a small molecule. O Usually involves the breaking of a double bond.

Condensation Polymerization (Polycondensation) O Reactions in which small molecules (such as H 2 O, and HCl) are formed when the monomers combine.

Polymerization Video

Learning Target Checkpoint O How do you differentiate between condensation polymerization and addition polymerization?