Polymer Chemistry Part 1 Polymer Characteristics and Classifications











- Slides: 26

Polymer Chemistry Part 1 Polymer Characteristics and Classifications

Definitions n. Polymer – A very long molecule composed of repeating units connected by covalent bonds n. Monomer – A repeated unit in a polymer. The reactant for the polymerization reaction.

Characterizing a Polymer n. Structure n. Classification n. Synthesis

Structure of a Polymer n. Skeletal Structure n. Chemical Structure

Skeletal Structure n. Linear – a chain with two ends

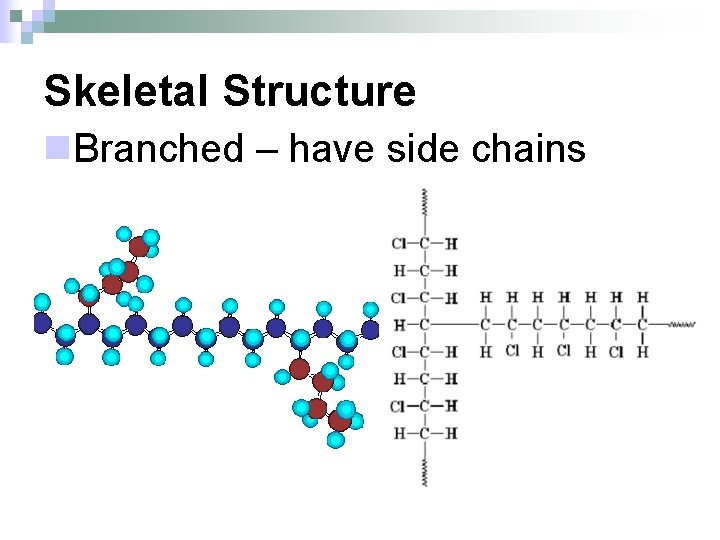
Skeletal Structure n. Branched – have side chains

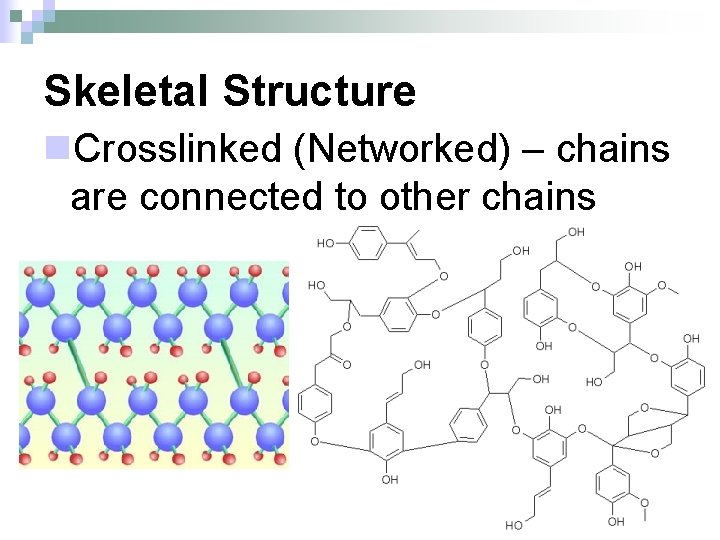
Skeletal Structure n. Crosslinked (Networked) – chains are connected to other chains

Chemical Structure n. Homopolymer – only one monomer (repeating unit) -A–A–A–A- n. Copolymer – more than one monomer

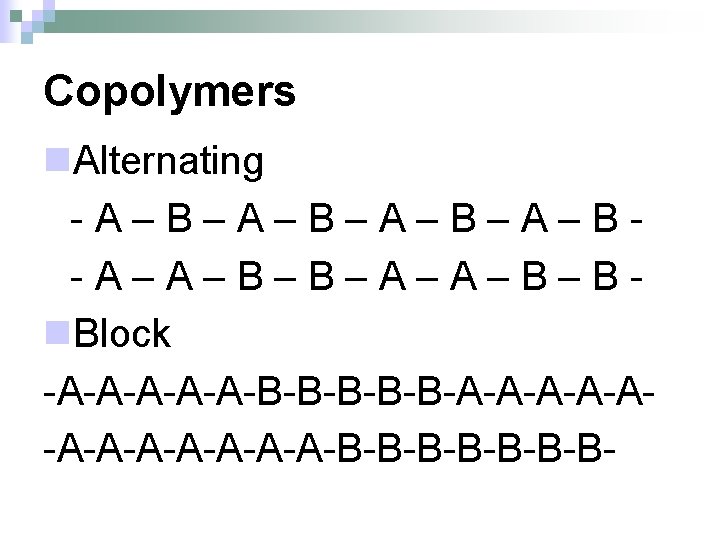
Copolymers n. Alternating -A–B–A–B–A–B-A–A–B–B–A–A–B–Bn. Block -A-A-A-B-B-B-A-A-A-A-A-A-B-B-B-B-

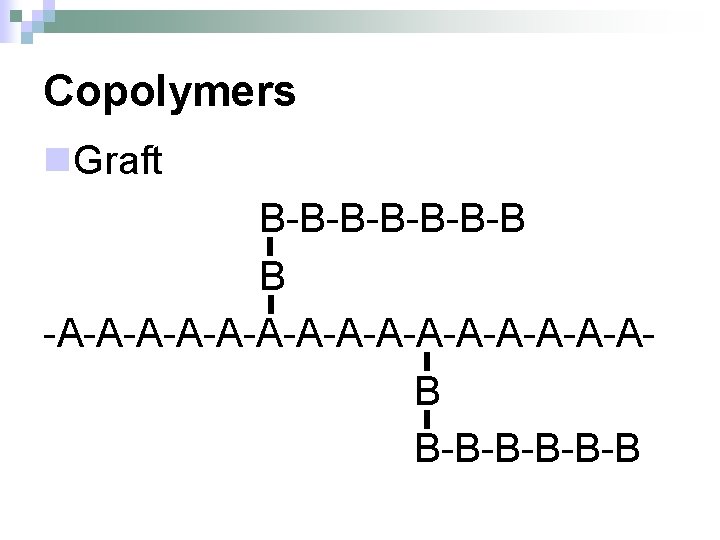
Copolymers n. Graft B-B-B-B B -A-A-A-A-A-A-A-AB B-B-B-B

Classifications n. Thermoplastic n. Elastomer n. Thermoset

Thermoplastics n. Linear or branched polymers which can be melted when heat is applied. n. Can be molded into any shape with processing techniques such as injection molding or extrusion. n. Most common “plastics”

Thermoplastics n. Plastics – bottles, grocery bags, water piping, rope, fishing line, car parts n. Most are recyclable n. Natural thermoplastics – silk, cellulose (proteins), polylactic acid

Codes for Plastics 1 n 1 – PETE – soft drink bottles n 2 – LDPE – plastic bags, toys n 3 – PVC – water pipes n 4 – HDPE – milk jugs n 5 – PP – bottle caps n 6 – PS – styrofoam

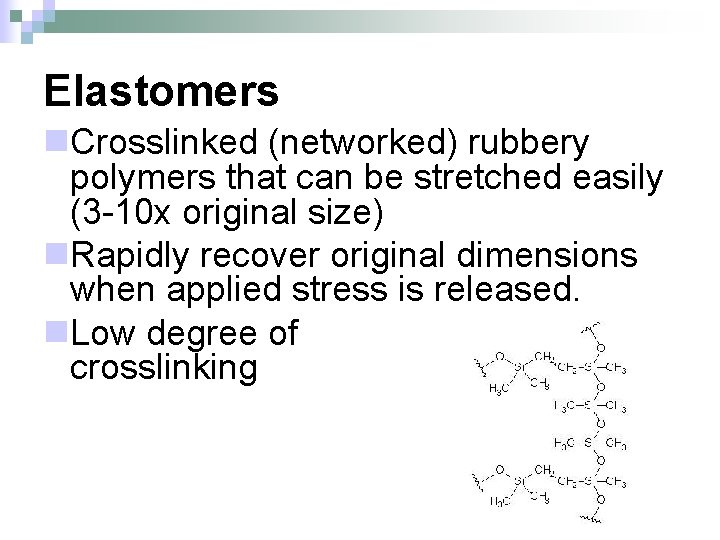
Elastomers n. Crosslinked (networked) rubbery polymers that can be stretched easily (3 -10 x original size) n. Rapidly recover original dimensions when applied stress is released. n. Low degree of crosslinking

Elastomers n. Uses – examination gloves, rubber bands, bouncing balls n. Not recyclable ¨Degrades (burns/scorches) when heat is added n. Natural elastomers – natural rubber, latex

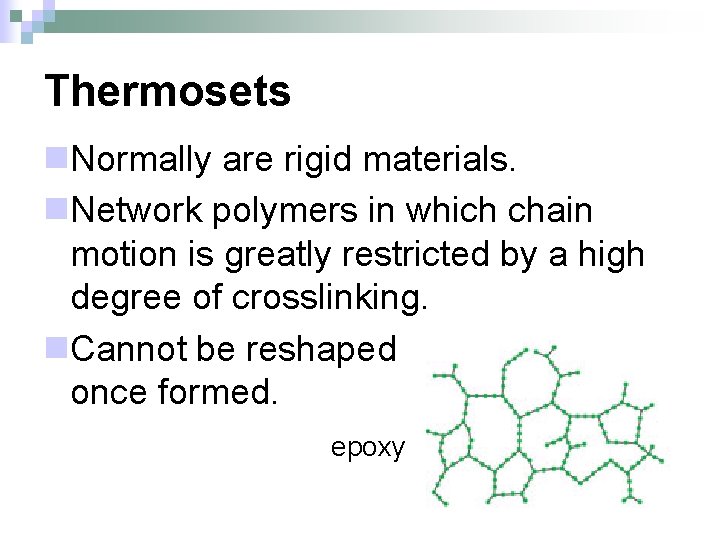
Thermosets n. Normally are rigid materials. n. Network polymers in which chain motion is greatly restricted by a high degree of crosslinking. n. Cannot be reshaped once formed. epoxy

Thermosets n. Uses – high temperature electrical applications, super glue, counter top laminates, epoxy resins, tires (vulcanized rubber) n. Cannot be recycled (burn/scorch with heat) n. Natural* thermosets – vulcanized rubber

Polymer Chemistry Part 2 Polymer Synthesis

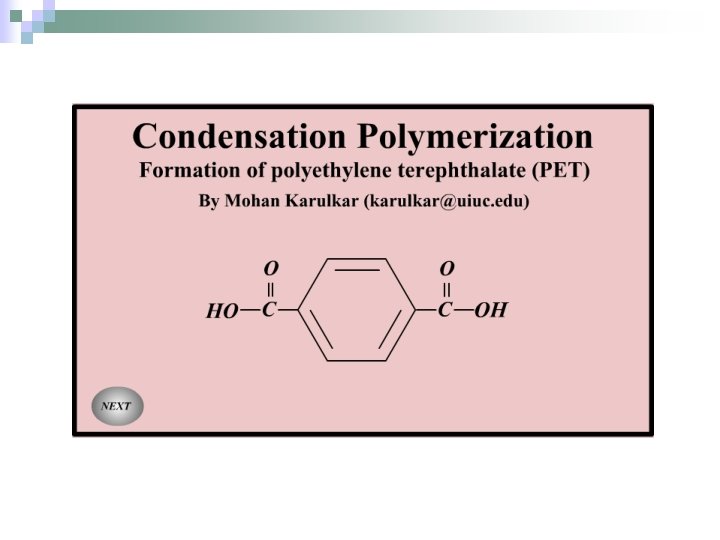
Polycondensation (Condensation Polymerisation) n. Reactions in which small molecules (H 2 O, HCl) are eliminated when the monomers combine.


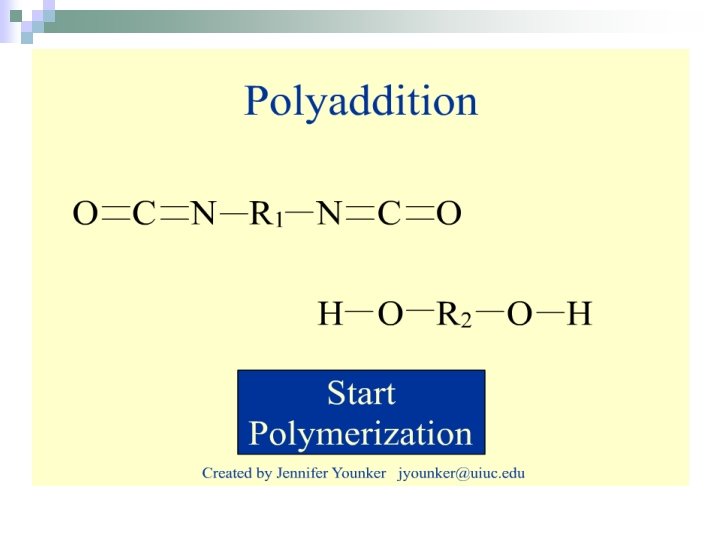
Polyaddition (Addition Polymerisation) n. Reactions in which monomers combine without the elimination of a small molecule. ¨Usually involves the breaking of a double bond.


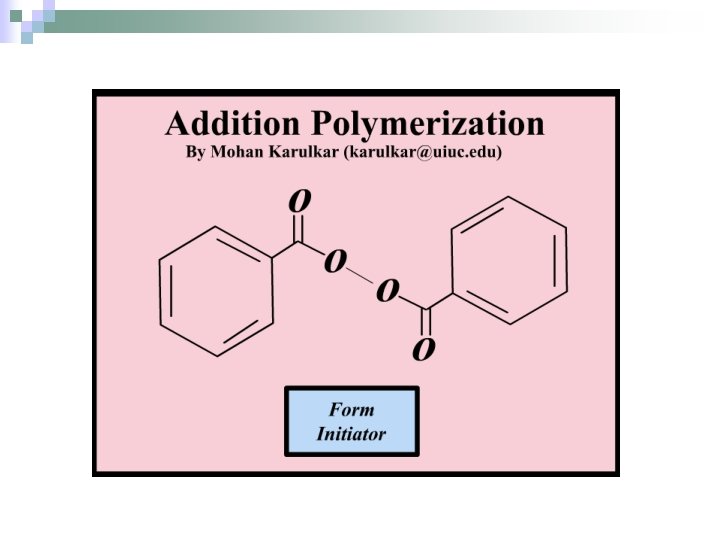
Polyaddition with Radicals n. Initiation – Creation of an active site (free radical). n. Propagation – Growth of polymer chain by addition of a monomer to an active site and the creation of a new active site.

Polyaddition with Radicals n. Termination – Growth of chain stops. ¨Combination – Two growing chains collide. ¨Disproportionation – A hydrogen atom is added to the end of a growing chain.
