Polymer Chemistry Part 1 OER Created Presented by
Polymer Chemistry (Part 1) OER Created & Presented by Dr. Rajdip Dey Techno India Salt Lake Kolkata
Polymers Content: z. Introduction: What are they? z. Types of Polymers z. Uses and Applications of Polymers z. Summary
Polymers - What are they? z. Polymers are a special kind of macromolecule z. The word polymer comes from the Greek words “poly, ” meaning “many”, and “meres, ” meaning “parts” or “repeating units” z. A Polymer consists of a large chain of repeating molecules (monomers) that are attached in an end to end fashion
Sources of Polymers z Oil (carbon) y 4% of crude oil is used for plastics z Sustainable sources (biopolymers) y. Wheat & corn y. Carrot z Recycling y. Difficult: all recycled items must be of the same polymer y. Mixed plastics can be used for low level products such as road surfacing, wood replacement

Description of Polymers z. Imagine a string of beads y. Each bead is identical (for example, red sphere) • Represents the “mer” y. The string can contain 100’s of beads • Represents the “poly” characteristic y. The string in between the beads represents the chemical bond between monomers
Length of Polymers z. Polymer chains are HUGE! z. Polymers typically consist of between 20, 000 and 40, 000 individual monomers y. If each bead on the string of beads were one inch apart, one polymer molecule could be as long as 10 football fields!!! z. This chain length is what gives the polymer most of its desirable characteristics
Description of Polymers z. Polymer chains are flexible, and usually “clump” together into a smaller shape z. This enables the individual chains to interact and become entangled z. This helps to give a polymer its strength and flexibility
Types of Polymers z. There are two main types of polymers y. Natural • (cotton, silk, wood, leather…) y. Synthetic • (plastics, nylon, latex…)
Synthetic Polymers z. There are two basic types of synthetic polymers y. Thermoplastics (plastics, Styrofoam) • These can be softened by heating and hardened by cooling - easily recycled • Can easily be cast into various shapes y. Thermosets (epoxy’s, adhesives) • These harden after being heated • Can easily be cast into different shapes • Cannot be reformed
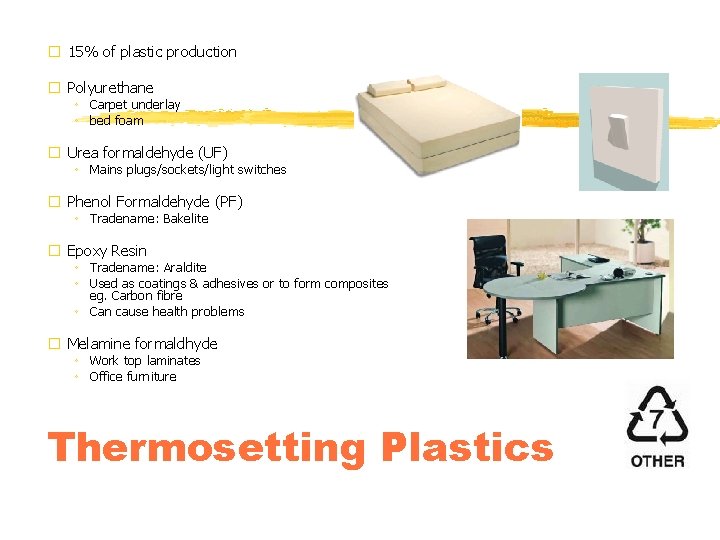
� 15% of plastic production � Polyurethane ◦ Carpet underlay ◦ bed foam � Urea formaldehyde (UF) ◦ Mains plugs/sockets/light switches � Phenol Formaldehyde (PF) ◦ Tradename: Bakelite � Epoxy Resin ◦ Tradename: Araldite ◦ Used as coatings & adhesives or to form composites eg. Carbon fibre ◦ Can cause health problems � Melamine formaldhyde ◦ Work top laminates ◦ Office furniture Thermosetting Plastics
Types of Polymers Copolymers z. Most polymer chains are made up of one type of monomer (for example, red beads) z. However, some polymers are made up of different types of monomers (for example, blue and red beads) - these are called copolymers

Copolymers z. There are four main types of copolymers z. The different monomers can be arranged either in an alternating or random fashion y. Alternating. . . -red-blue-red-blue-red-… y. Random …-blue-red-blue-red-blue-…

Copolymers z. There also block copolymers and graft co-polymers y. Block …-R-R-R-B-B-B-R-R-R-… y. Graft …-R-R-R-R-R-R-R-R-R-… | B-B-B-B-B-…
Polymers - Pros and Cons z. A polymer has many very advantageous properties. Polymers are: y. Lightweight y. Strong and durable y. Cheap y. Easy to manufacture z. Unfortunately, polymers do not easily biodegrade and end up producing large amounts of waste
Uses of Polymers z. Polymers are incorporated into nearly every aspect of daily life y. Entertainment y. Sports y. Clothes y. Hobbies/Toys y. Household products y. Automotive
Carbon Based Polymers z. Poly. Propylene (PP) y. Tupperware (lunch boxes) z. Poly Vinyl Chloride (PVC) y. Window frames z. Poly. Styrene (PS) y. Packaging y. Yoghurt pots / vending machine cups

Carbon Based Polymers z Acrylic y Paint y Point of sale displays y Baths y Car lights z HDPE (High Density PE) y Bottles (biggest application) y milk bottles (largest bottle sector) z LDPE (low Density PE) y Supermarket carrier bags y Packaging film (eg. cling film) y Washing up liquid bottles z PET (PE Terephthalate) y fizzy drinks bottles ◦ Carbonation makes HDPE unsuitable y Space blankets z ABS (Acrylonitrile Butadiene Styrene) y Car batteries y Calculators / mobile phones y Safety helmets

Material Properties: ABS z z z z Amorphous Good resistance against medium temperatures (< 1000 C) Hard tough antistatic. good resistance against chemicals. Poor resistance to UV-light Can be painted z Min temp: z Max Temp: z Glass Temp: -250 C 800 C 1100 C

Material Properties: PET � Very light � AKA: Polyester � Can stand high tensile stress ◦ Often used for magnetic tape � � � hard, stiff, strong dimensionally stable absorbs very little water good chemical resistance except to alkalis Medium resistance to UV most commonly recycled plastic ◦ drinks bottles are made from PET � Semi-crystaline ◦ Must be rapid cooled to make it amorphous & transparent � Can degrade & become discoloured during heat treatment � Adds an unwanted flavour to food (can be compensated for at addition cost) � � Min Temp: Max Temp: Glass transition temperature: Melting point: -500 C 1700 C 82 o. C. 250 o. C.
Polymer Chemistry z. A polymer chain is built on a Carbon backbone z. A monomer unit consists of a small carbon chain attached to a specific type of functional group z. The functional group is what gives each polymer chain its individual characteristics
Polymer Chemistry z. As previously stated, polymer chains interact with one another, becoming entangled z. Polymer chains also form cross-links with adjacent chains, which allows the polymer to hold its shape and gives added strength

Summary z. Polymers are made up of large chains of repeating units, called monomers z. Individual chains interact to form a stronger overall substance through entanglements and cross-links z. Polymers are incorporated into almost every aspect of daily life z. Polymers are lightweight, strong, and inexpensive
- Slides: 22