Polymer Chemistry ControlledLiving Polymerization Donghui Zhang Fall 2012

![Solvent effect on anionic polymerizations Ke [M] kp {M-n} [M-] propagating 16 Solvent effect on anionic polymerizations Ke [M] kp {M-n} [M-] propagating 16](https://slidetodoc.com/presentation_image_h/962707c179e87275204a1f00155f9396/image-16.jpg)

![Ring Opening Metathesis Polymerization (ROMP) [Ru] or [Mo] or [W] catalyst Schrock’s catalyst 2 Ring Opening Metathesis Polymerization (ROMP) [Ru] or [Mo] or [W] catalyst Schrock’s catalyst 2](https://slidetodoc.com/presentation_image_h/962707c179e87275204a1f00155f9396/image-55.jpg)

- Slides: 73

Polymer Chemistry Controlled/Living Polymerization Donghui Zhang Fall 2012 1

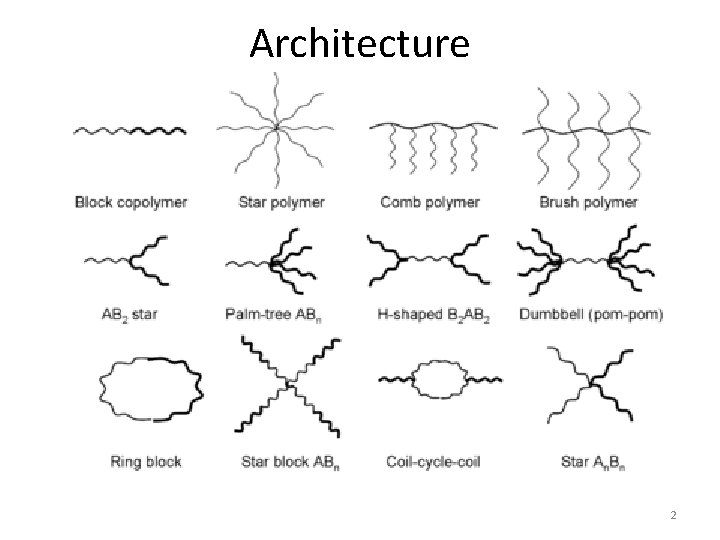
Architecture 2

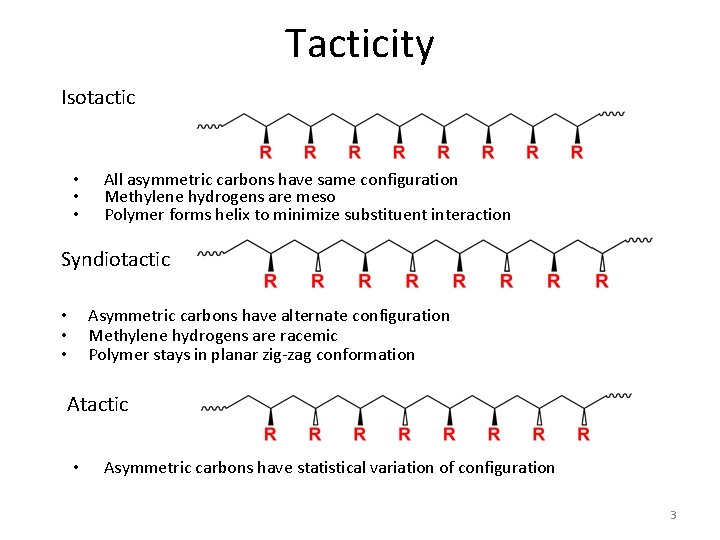
Tacticity Isotactic • • • All asymmetric carbons have same configuration Methylene hydrogens are meso Polymer forms helix to minimize substituent interaction Syndiotactic Asymmetric carbons have alternate configuration Methylene hydrogens are racemic Polymer stays in planar zig-zag conformation • • • Atactic • Asymmetric carbons have statistical variation of configuration 3

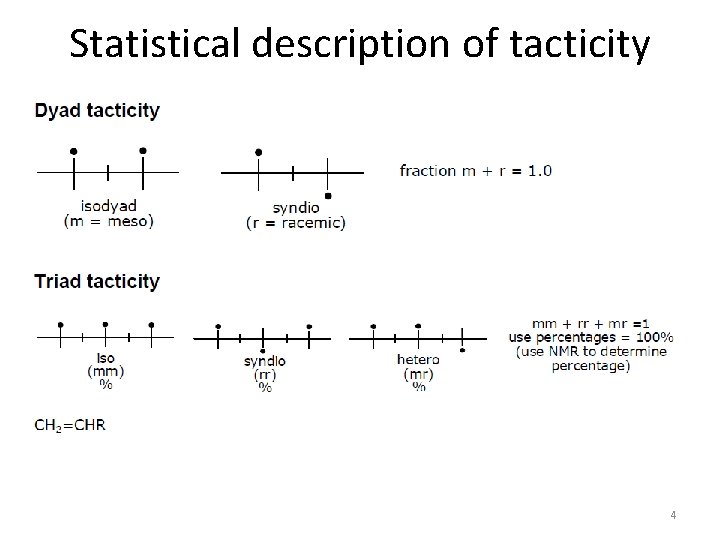
Statistical description of tacticity 4

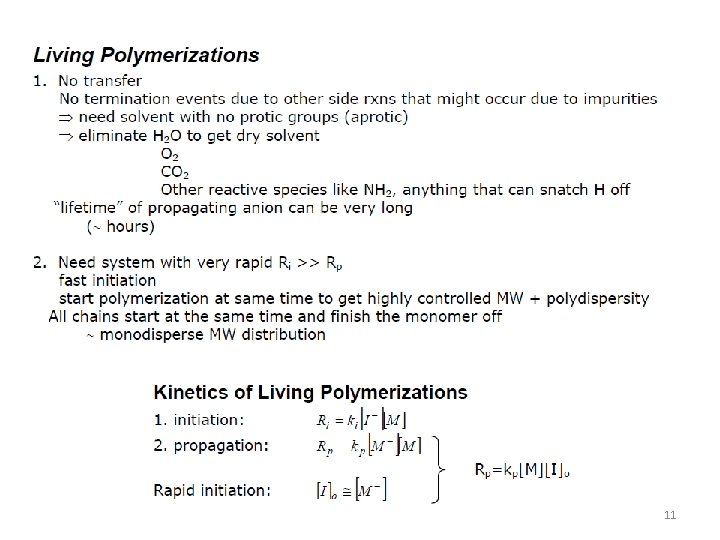
Major Developments in the 1950 -60's Living Polymerization (Anionic) • Mw/Mn 1 • Blocks, telechelics and stars available (Controlled molecular architecture) • Statistical Stereochemical Control • Statistical Compositions and Sequences • Severe functional group restrictions 5

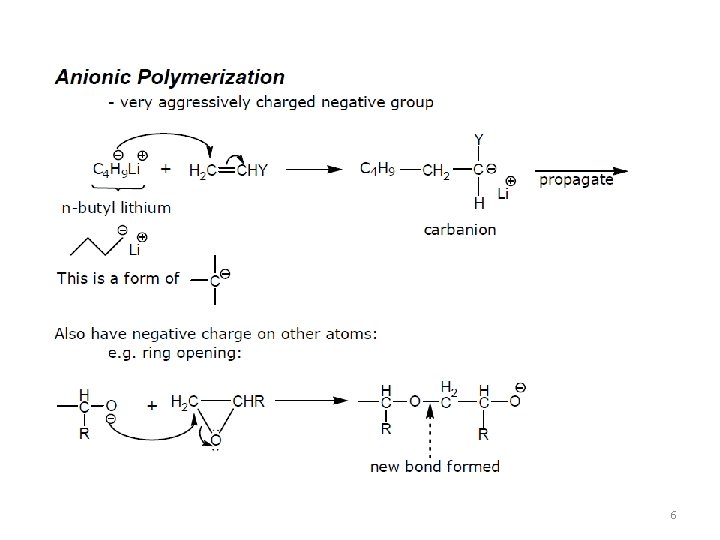
6

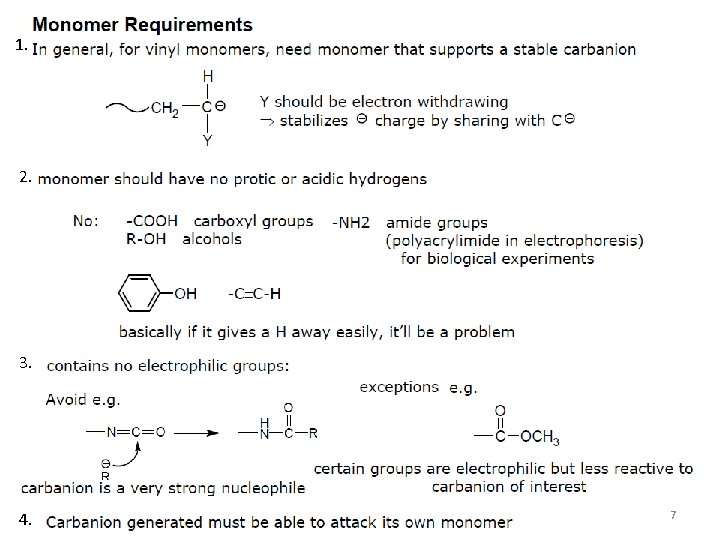
1. 2. 3. 4. 7

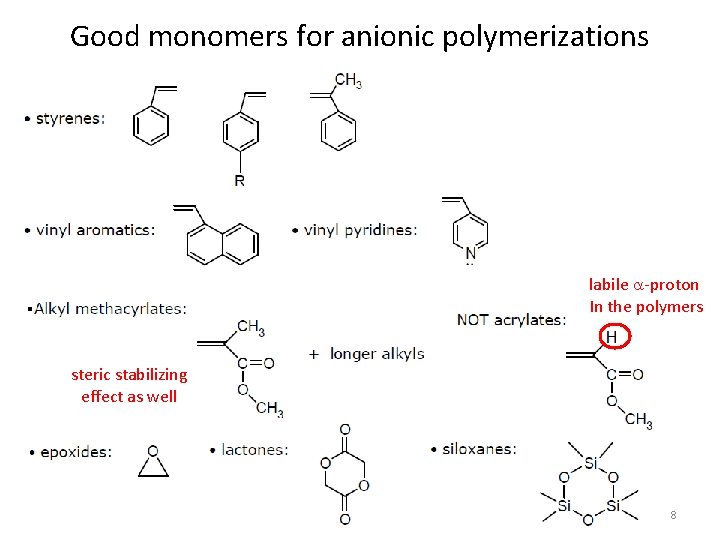
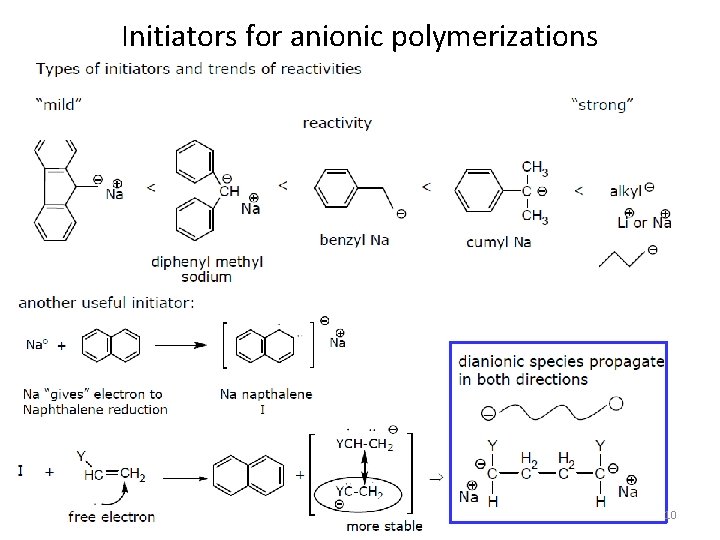
Good monomers for anionic polymerizations labile a-proton In the polymers . steric stabilizing effect as well 8

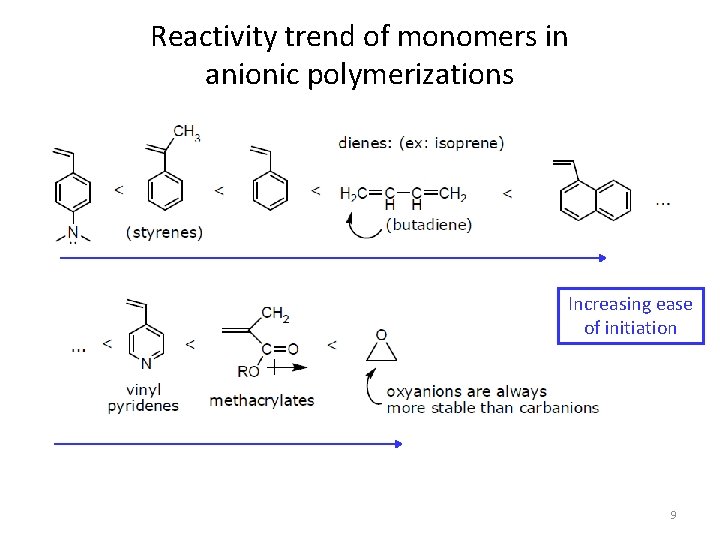
Reactivity trend of monomers in anionic polymerizations Increasing ease of initiation 9

Initiators for anionic polymerizations 10

11

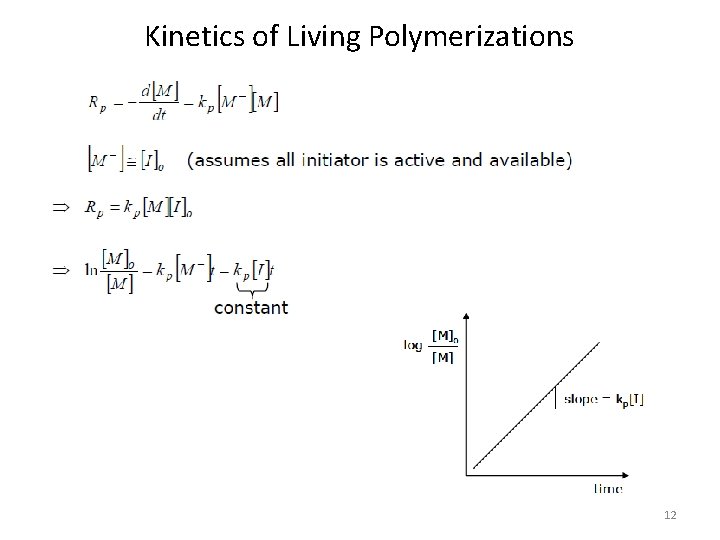
Kinetics of Living Polymerizations 12

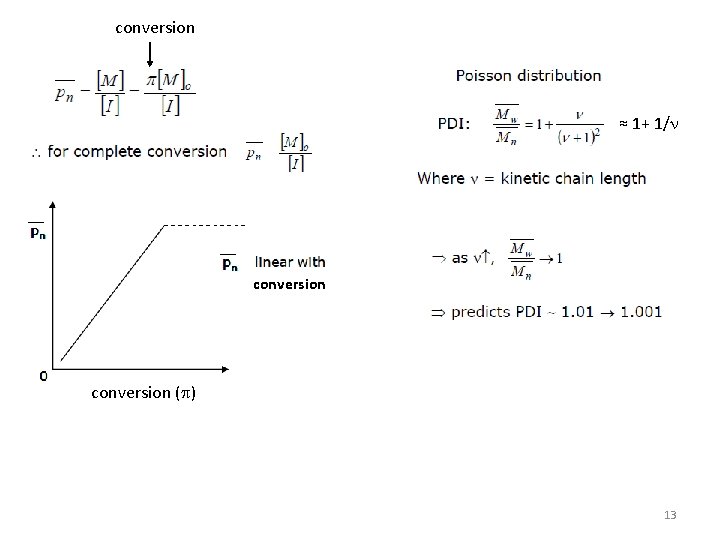
conversion ≈ 1+ 1/n conversion (p) 13

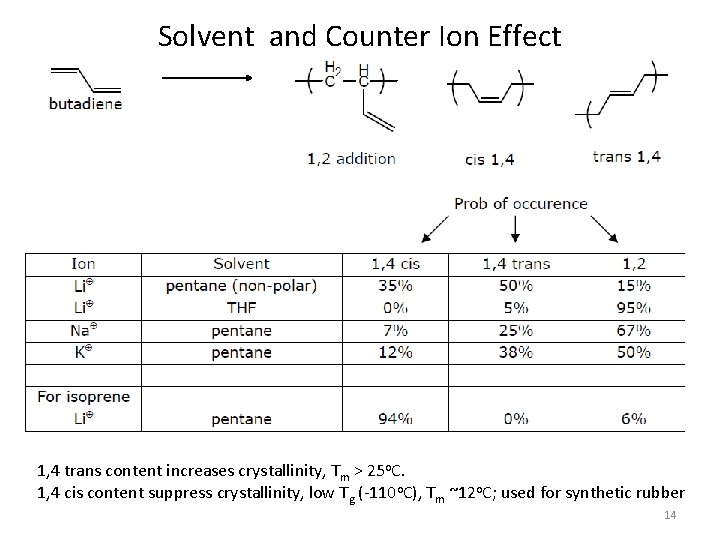
Solvent and Counter Ion Effect 1, 4 trans content increases crystallinity, Tm > 25 o. C. 1, 4 cis content suppress crystallinity, low Tg (-110 o. C), Tm ~12 o. C; used for synthetic rubber 14

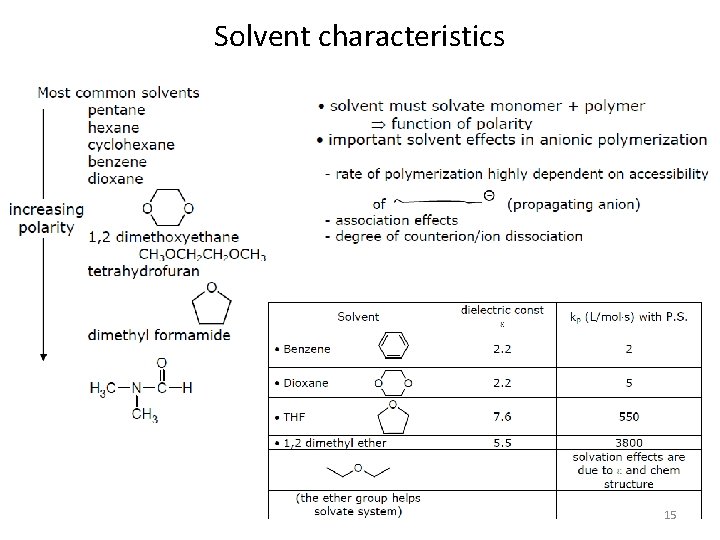
Solvent characteristics 15
![Solvent effect on anionic polymerizations Ke M kp Mn M propagating 16 Solvent effect on anionic polymerizations Ke [M] kp {M-n} [M-] propagating 16](https://slidetodoc.com/presentation_image_h/962707c179e87275204a1f00155f9396/image-16.jpg)
Solvent effect on anionic polymerizations Ke [M] kp {M-n} [M-] propagating 16

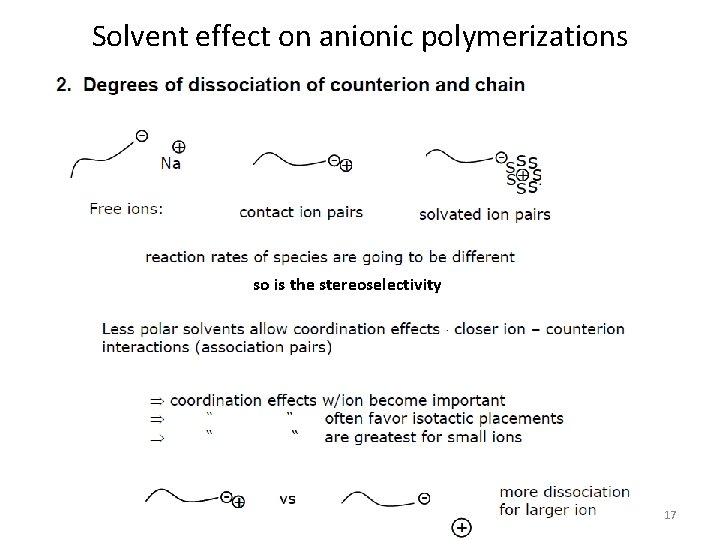
Solvent effect on anionic polymerizations so is the stereoselectivity 17

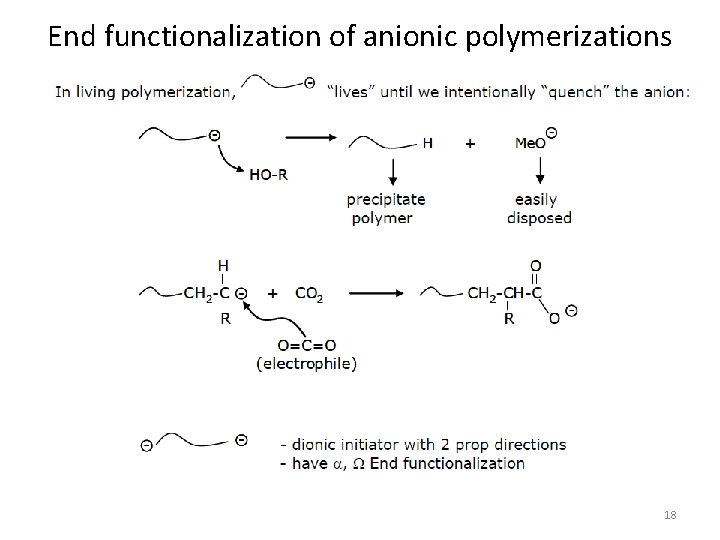
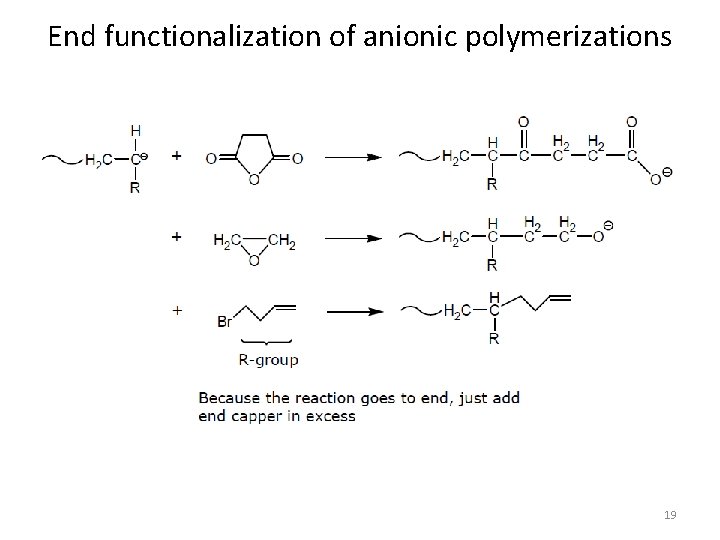
End functionalization of anionic polymerizations 18

End functionalization of anionic polymerizations 19

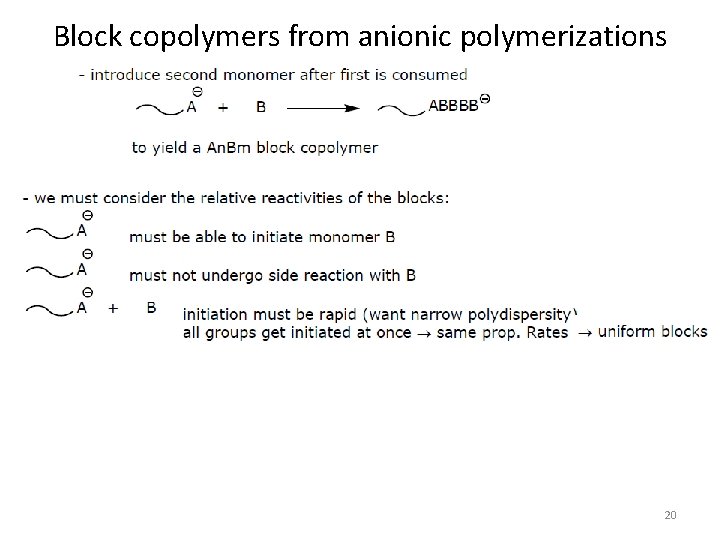
Block copolymers from anionic polymerizations 20

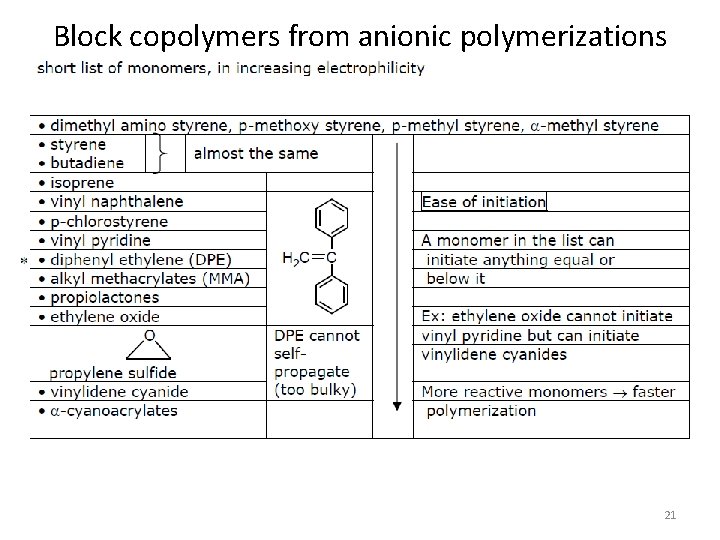
Block copolymers from anionic polymerizations 21

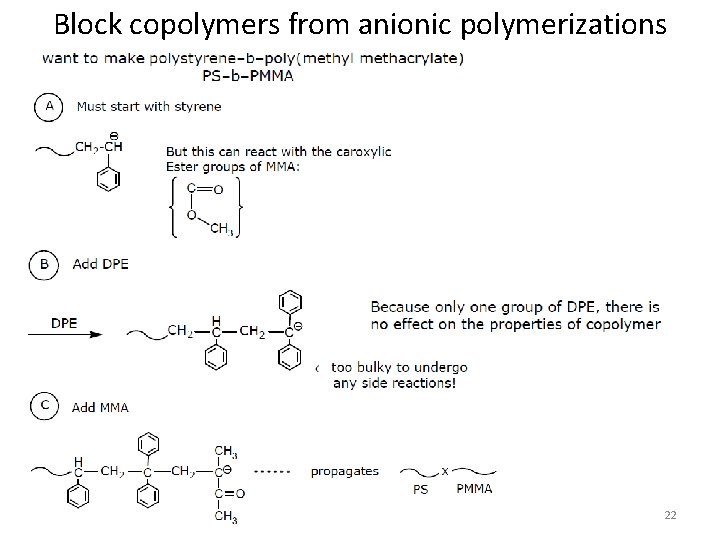
Block copolymers from anionic polymerizations 22

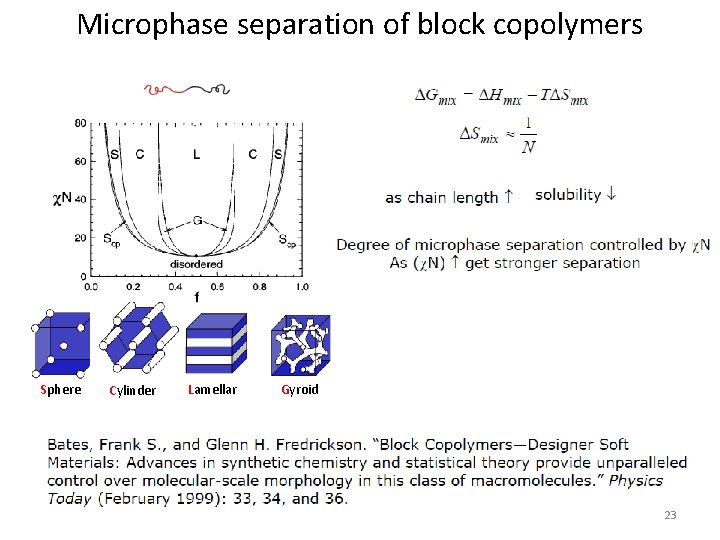
Microphase separation of block copolymers Sphere Cylinder Lamellar Gyroid 23

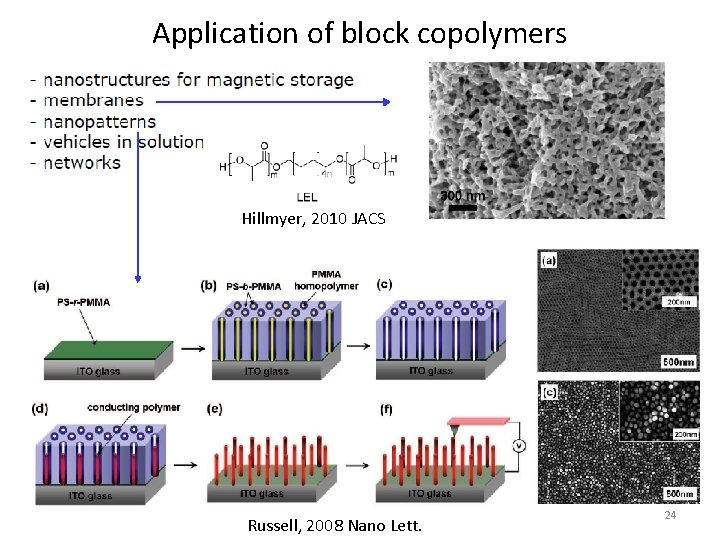
Application of block copolymers Hillmyer, 2010 JACS Russell, 2008 Nano Lett. 24

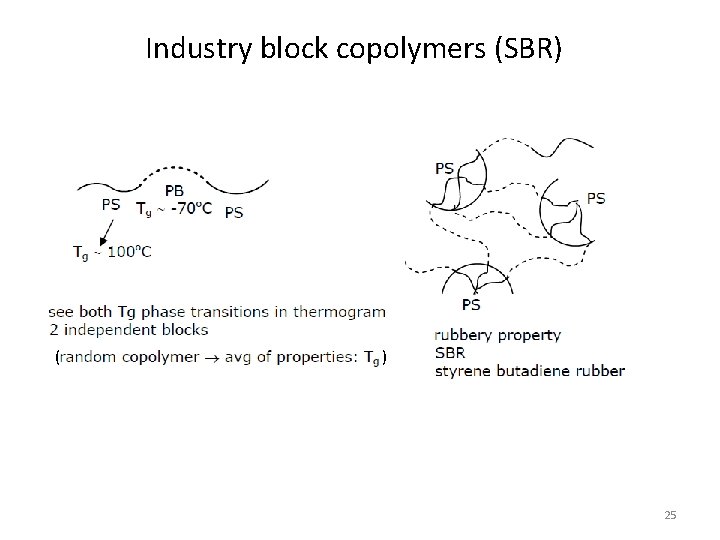
Industry block copolymers (SBR) ( ) 25

Additional Developments in the 1980's "Immortal" Polymerization (Cationic) – – – Mw/Mn 1. 05 Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences Severe functional group restrictions 26

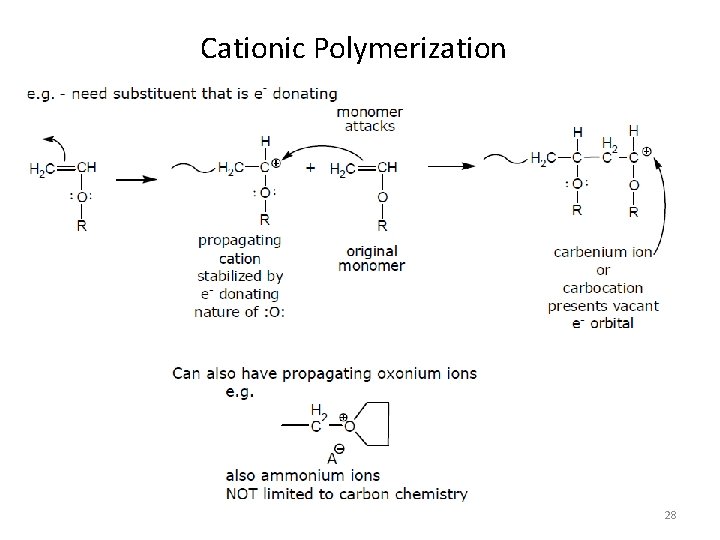
Cationic Polymerization 27

Cationic Polymerization 28

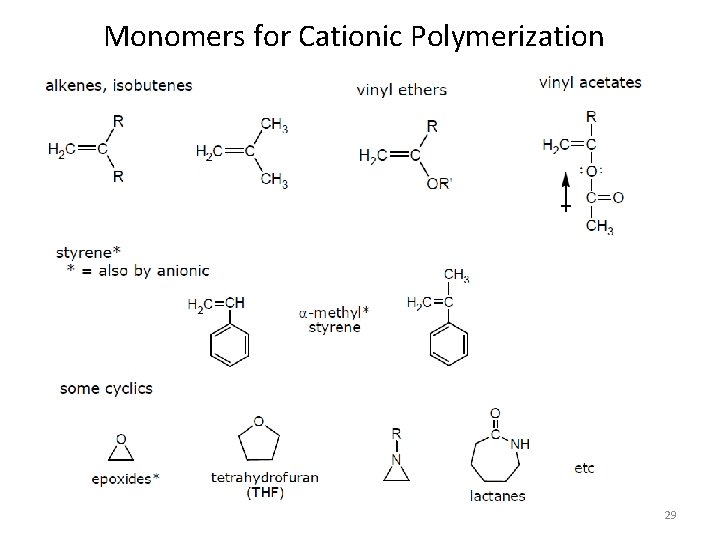
Monomers for Cationic Polymerization 29

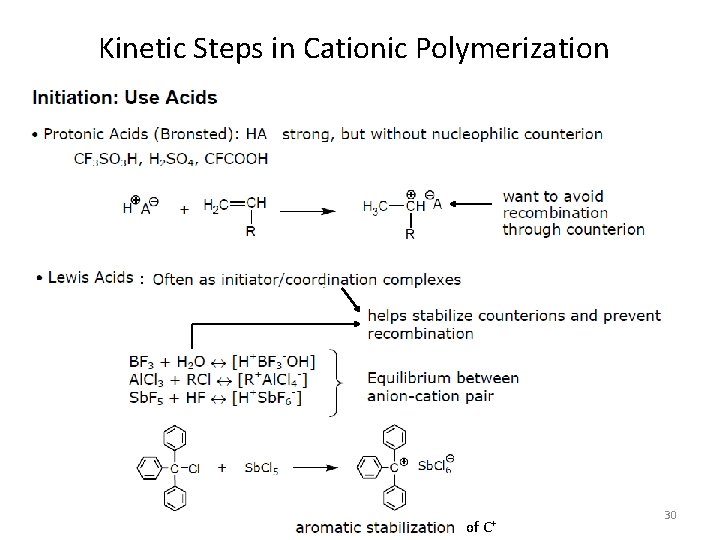
Kinetic Steps in Cationic Polymerization : of C+ 30

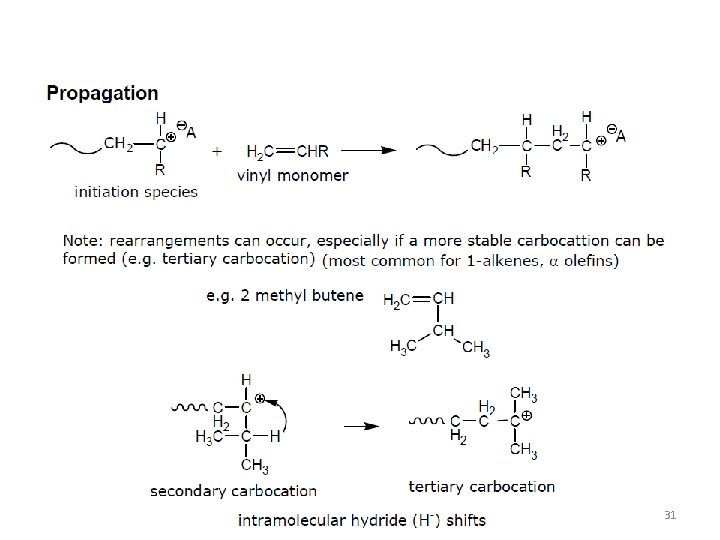
31

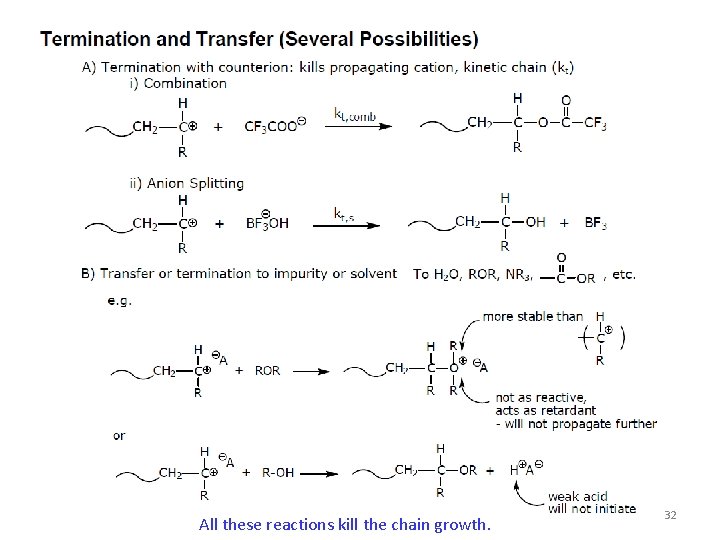
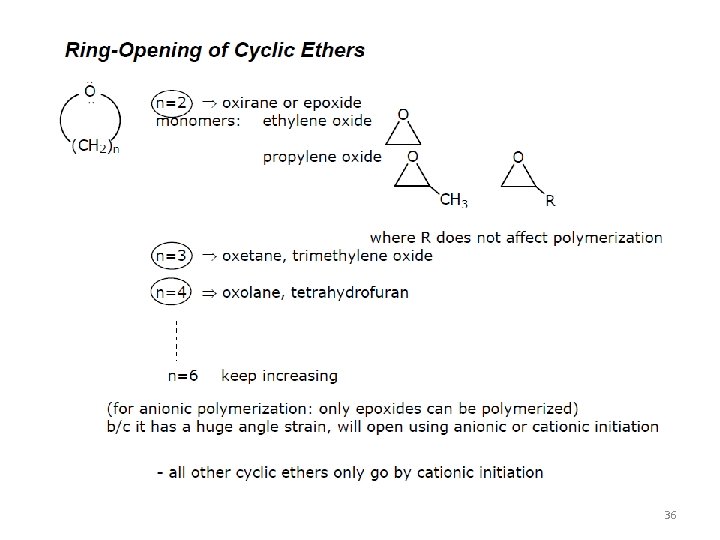
All these reactions kill the chain growth. 32

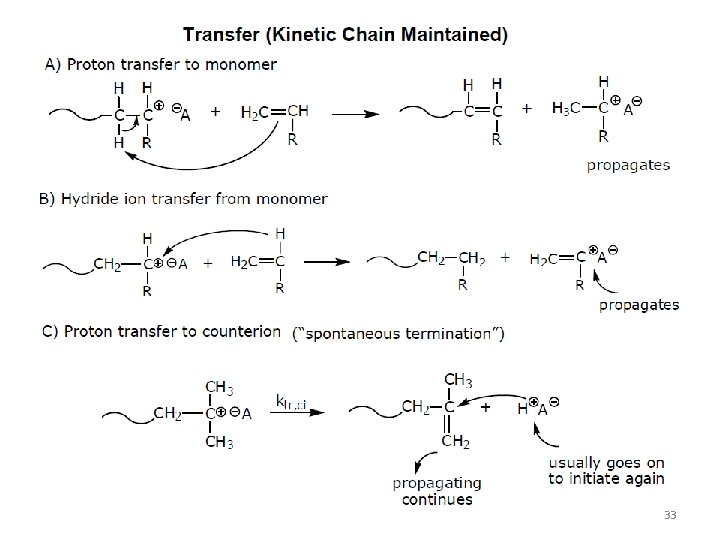
33

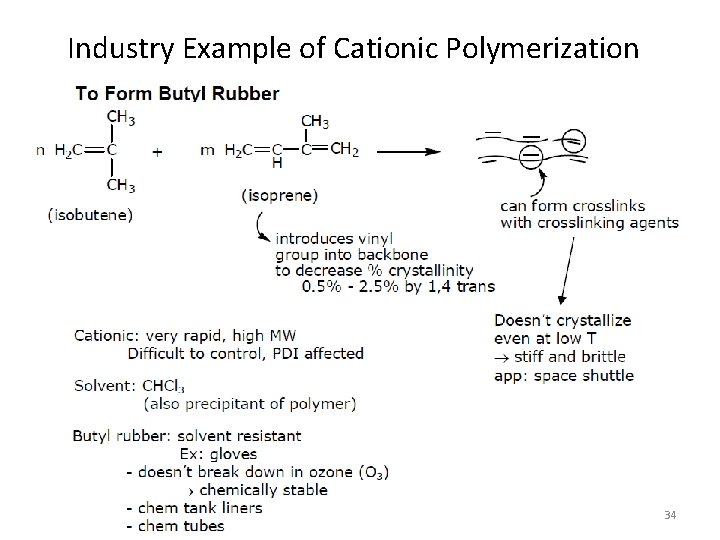
Industry Example of Cationic Polymerization 34

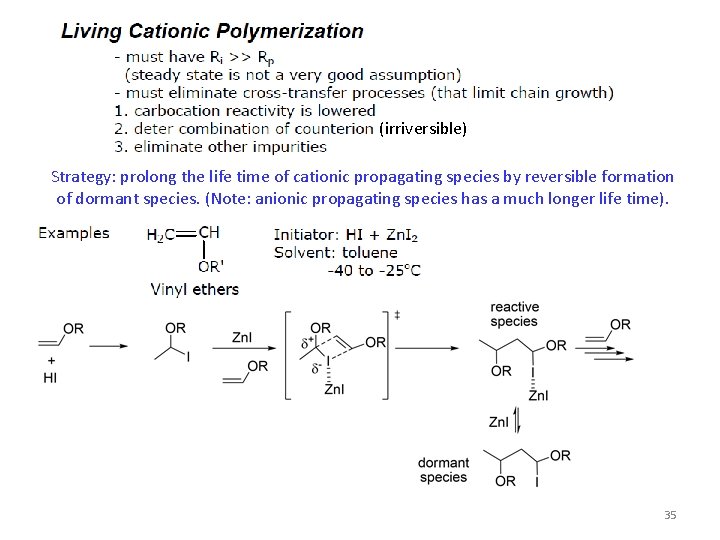
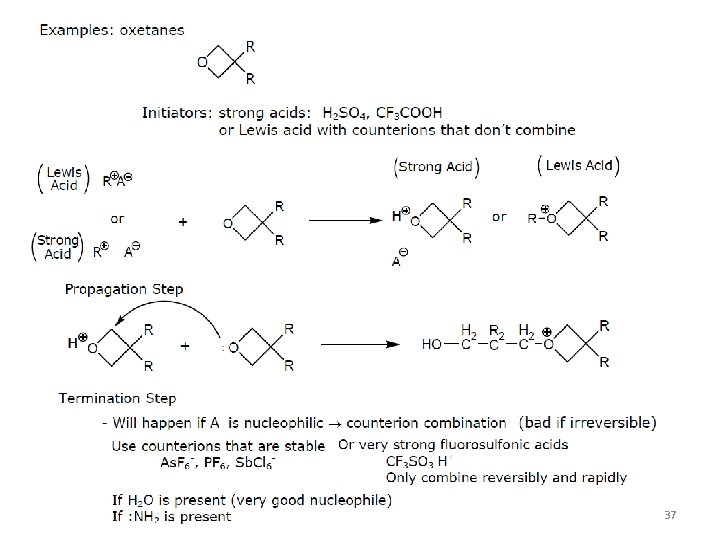
(irriversible) Strategy: prolong the life time of cationic propagating species by reversible formation of dormant species. (Note: anionic propagating species has a much longer life time). 35

36

37

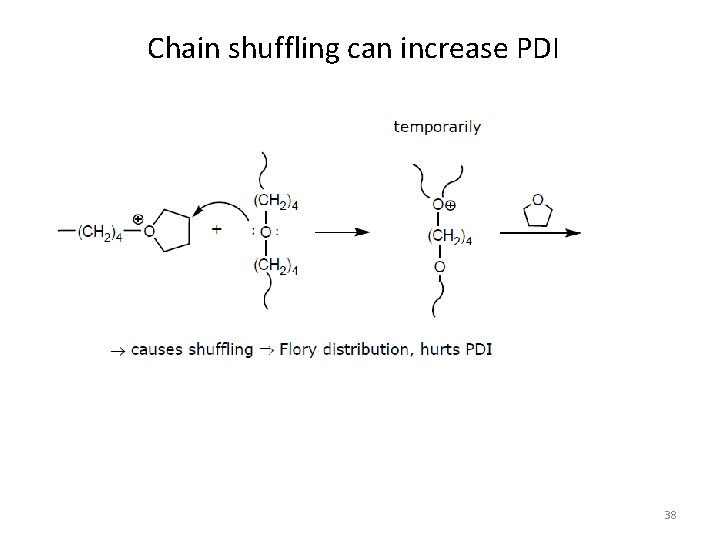
Chain shuffling can increase PDI 38

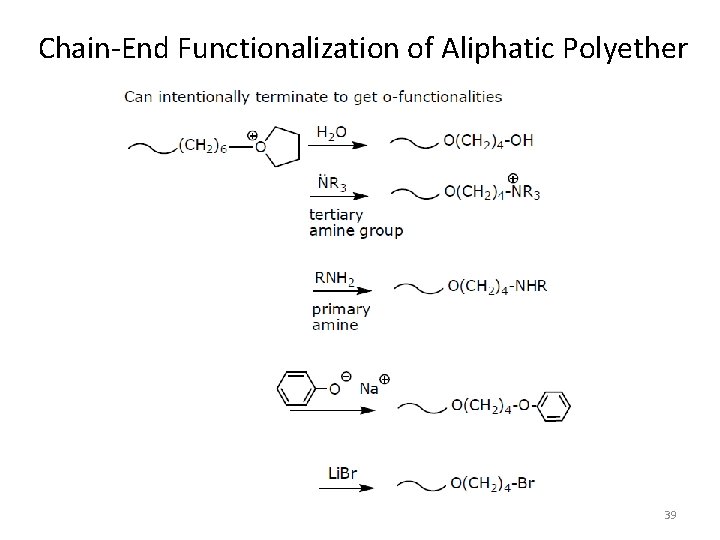
Chain-End Functionalization of Aliphatic Polyether 39

Free Radical Initiated Polymerization • • Controlled Free Radical Polymerization Broad range of monomers available Accurate control of molecular weight Mw/Mn 1. 05 --Almost monodisperse Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences 40

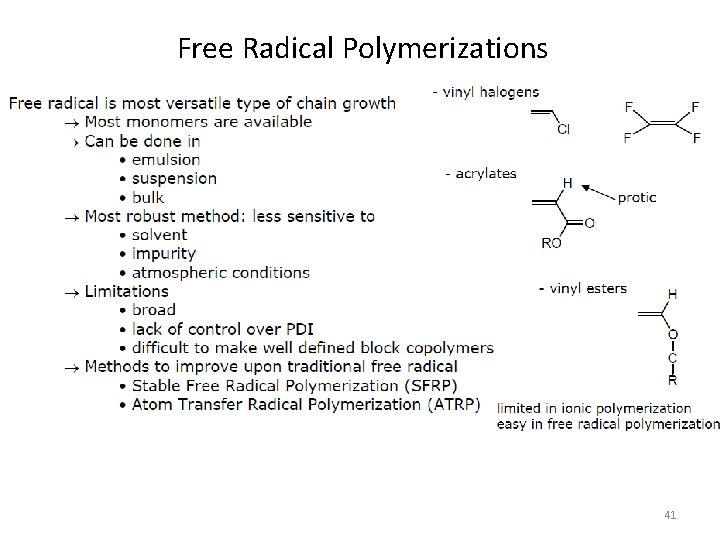
Free Radical Polymerizations 41

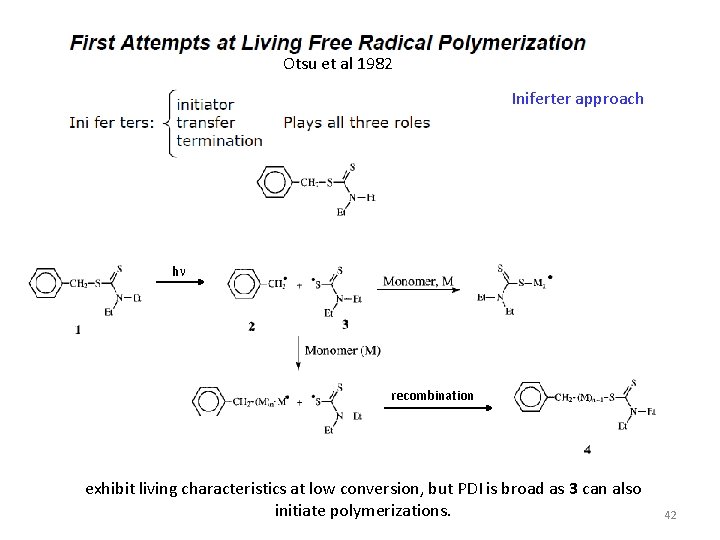
Otsu et al 1982 Iniferter approach hn recombination exhibit living characteristics at low conversion, but PDI is broad as 3 can also initiate polymerizations. 42

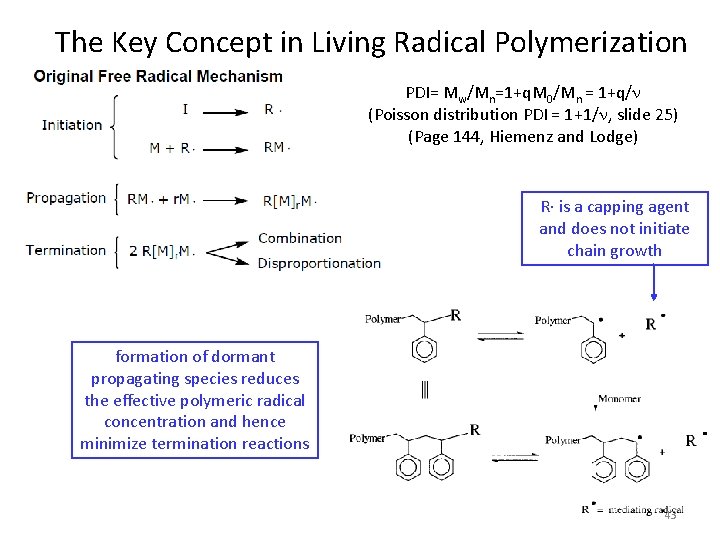
The Key Concept in Living Radical Polymerization PDI= Mw/Mn=1+q. M 0/Mn = 1+q/n (Poisson distribution PDI = 1+1/n, slide 25) (Page 144, Hiemenz and Lodge) R∙ is a capping agent and does not initiate chain growth formation of dormant propagating species reduces the effective polymeric radical concentration and hence minimize termination reactions 43

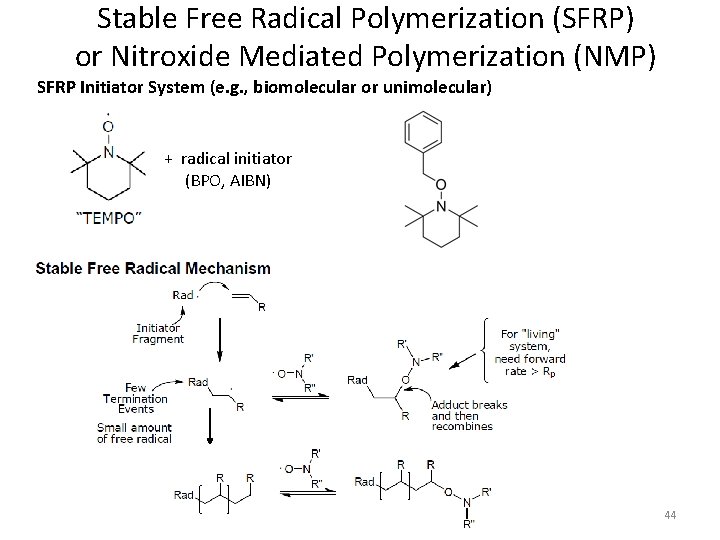
Stable Free Radical Polymerization (SFRP) or Nitroxide Mediated Polymerization (NMP) SFRP Initiator System (e. g. , biomolecular or unimolecular) + radical initiator (BPO, AIBN) 44

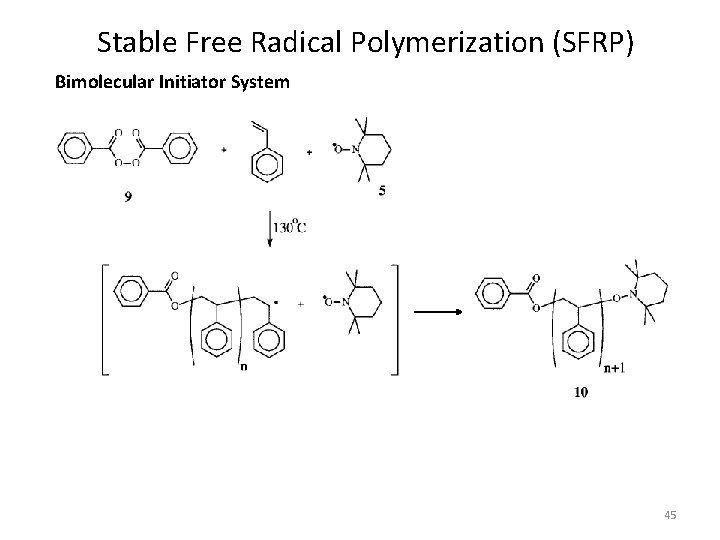
Stable Free Radical Polymerization (SFRP) Bimolecular Initiator System 45

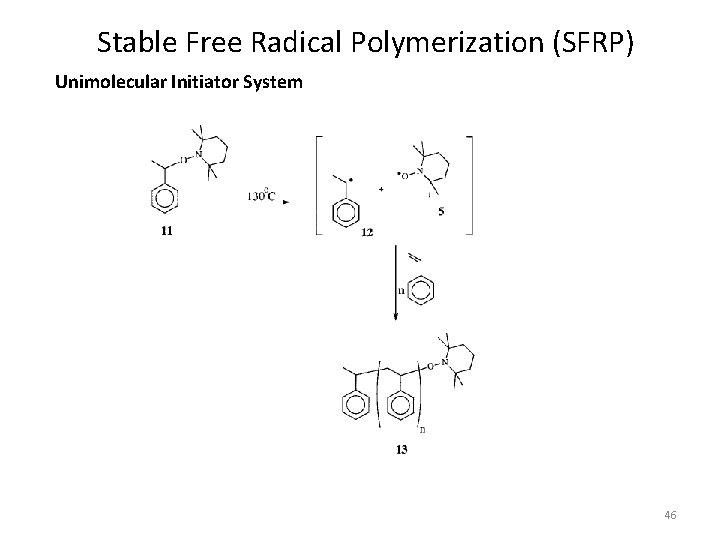
Stable Free Radical Polymerization (SFRP) Unimolecular Initiator System 46

State of Art for SFRP • MW > 105, PDI = 1. 1 -1. 2 • High reaction temperature (125 -145 o. C) • Long reaction time (24 -72 hr) • Low to moderate conversion (<70%) • Limited scope of monomers: St, MA, MMA etc. • functionalized alkoxyamine is required for block or telechelic polymer synthesis lower temperature (60 -80 o. C), shorter reaction time (several hrs) and higher conversion (>99%) are desired 47

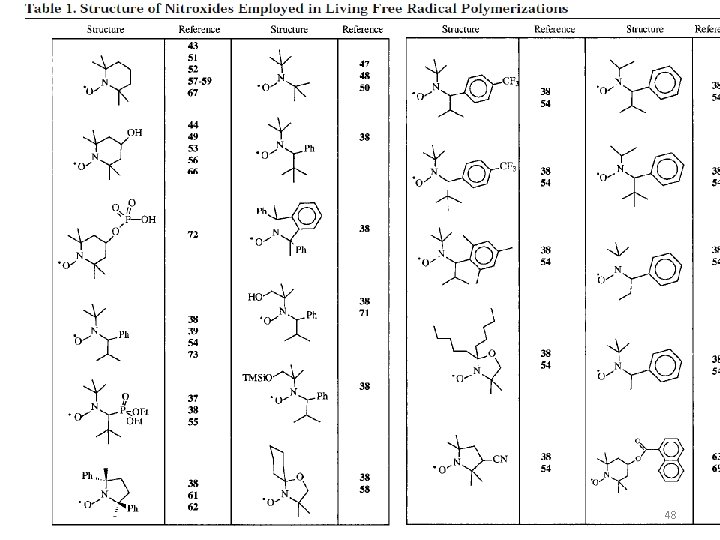
48

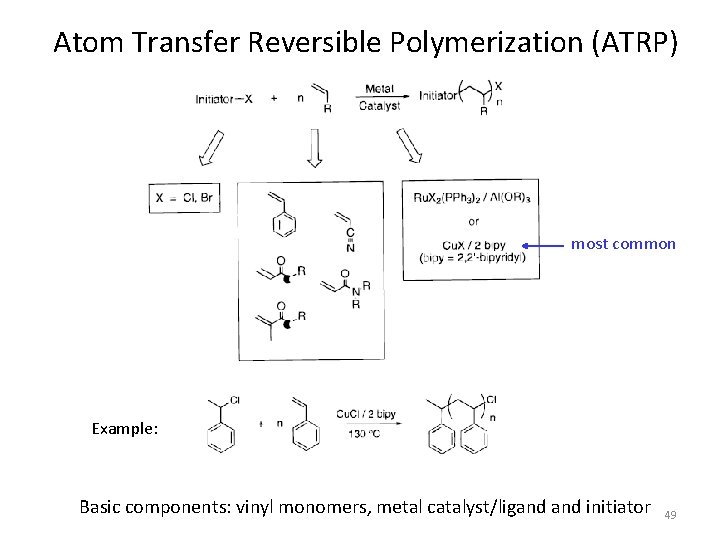
Atom Transfer Reversible Polymerization (ATRP) most common Example: Basic components: vinyl monomers, metal catalyst/ligand initiator 49

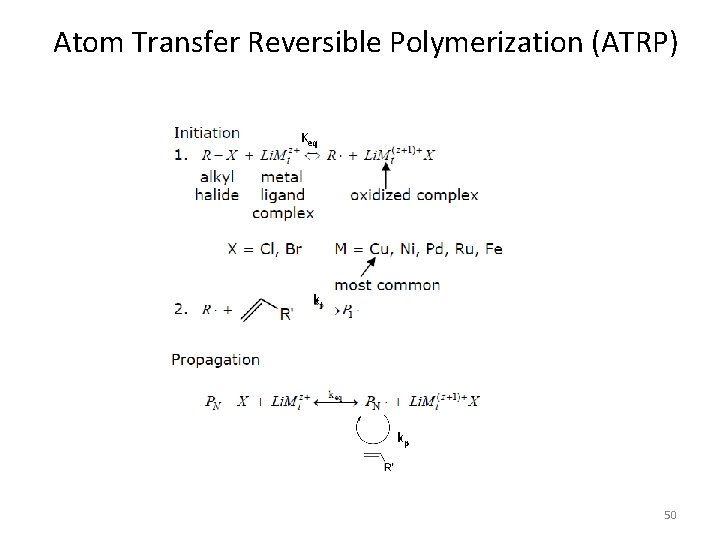
Atom Transfer Reversible Polymerization (ATRP) Keq ki Keq’ kp 50

State of Art for ATRP • MW > 105 easily, PDI = 1. 1 -1. 6 • reaction temperature (70 -130 o. C) • Low to moderate conversion (<80%) • Tolerant of functional groups, wide scope of monomers: St, MA, MMA, acrylamide, vinylpyridine (VP), acrylonitrile (AN) etc. (acrylic acid, vinyl halide, vinyl ether, a-olefin cannot be polymerized) • Availability of a variety of initiator and catalysts. • block polymers and telechelic polymers are readily prepared. • metal contaminant is sometime less desired 51

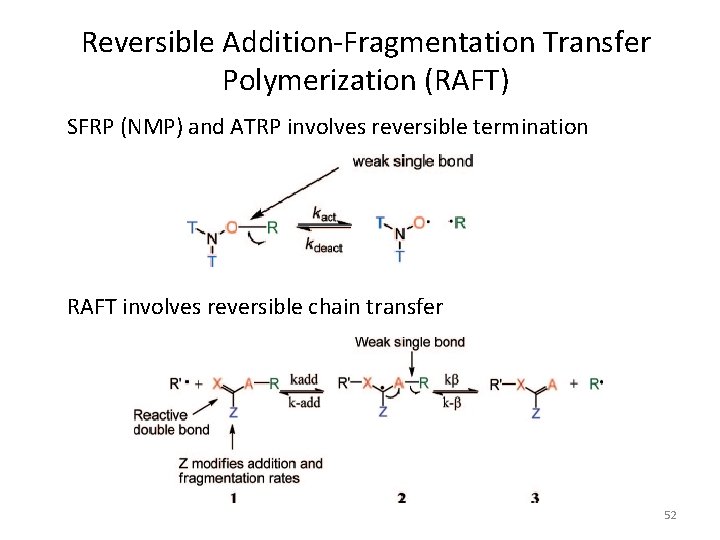
Reversible Addition-Fragmentation Transfer Polymerization (RAFT) SFRP (NMP) and ATRP involves reversible termination RAFT involves reversible chain transfer 52

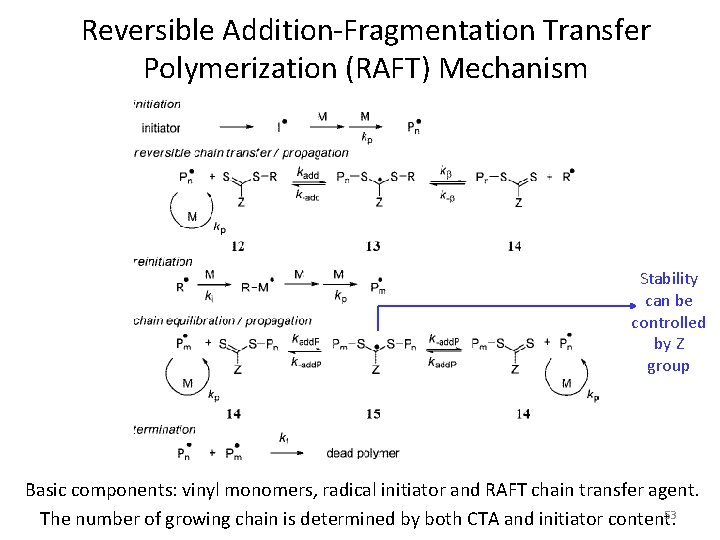
Reversible Addition-Fragmentation Transfer Polymerization (RAFT) Mechanism Stability can be controlled by Z group Basic components: vinyl monomers, radical initiator and RAFT chain transfer agent. 53 The number of growing chain is determined by both CTA and initiator content.

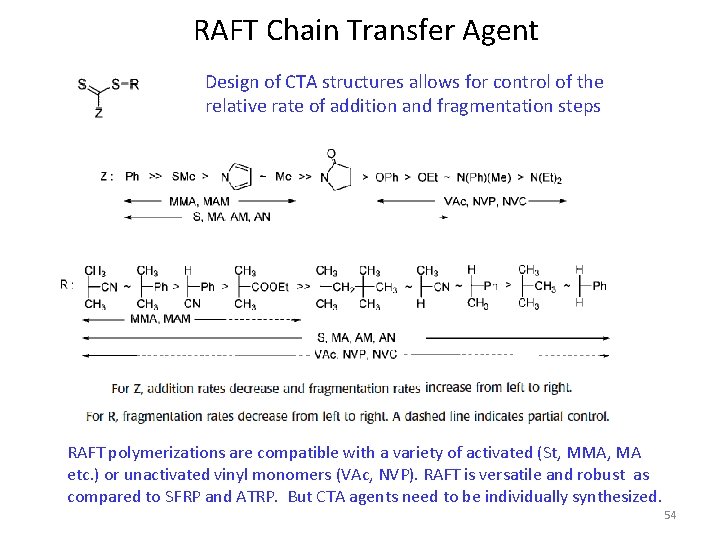
RAFT Chain Transfer Agent Design of CTA structures allows for control of the relative rate of addition and fragmentation steps RAFT polymerizations are compatible with a variety of activated (St, MMA, MA etc. ) or unactivated vinyl monomers (VAc, NVP). RAFT is versatile and robust as compared to SFRP and ATRP. But CTA agents need to be individually synthesized. 54
![Ring Opening Metathesis Polymerization ROMP Ru or Mo or W catalyst Schrocks catalyst 2 Ring Opening Metathesis Polymerization (ROMP) [Ru] or [Mo] or [W] catalyst Schrock’s catalyst 2](https://slidetodoc.com/presentation_image_h/962707c179e87275204a1f00155f9396/image-55.jpg)
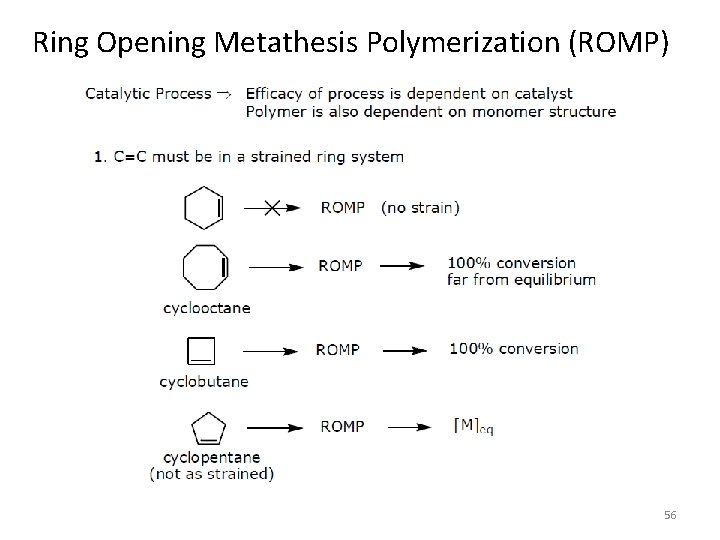
Ring Opening Metathesis Polymerization (ROMP) [Ru] or [Mo] or [W] catalyst Schrock’s catalyst 2 nd Gen. Grubb’s catalyst 55

Ring Opening Metathesis Polymerization (ROMP) 56

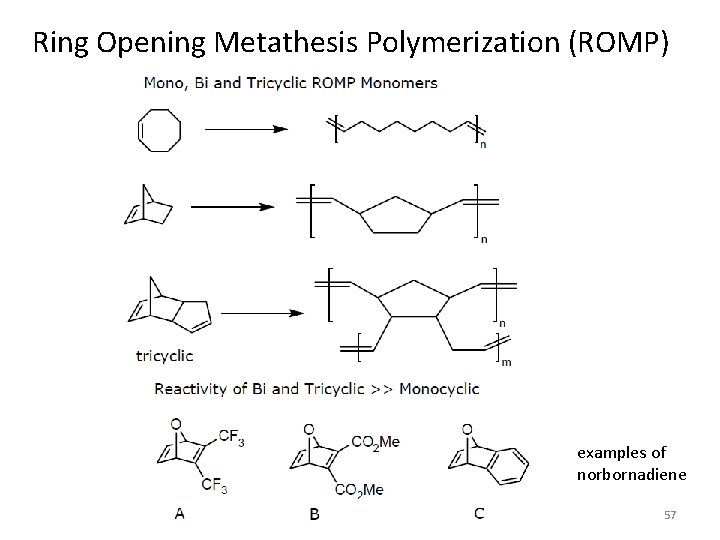
Ring Opening Metathesis Polymerization (ROMP) examples of norbornadiene 57

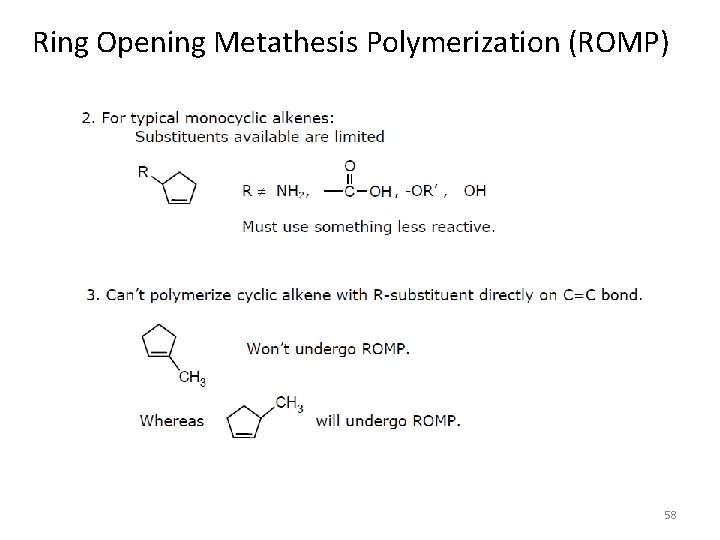
Ring Opening Metathesis Polymerization (ROMP) 58

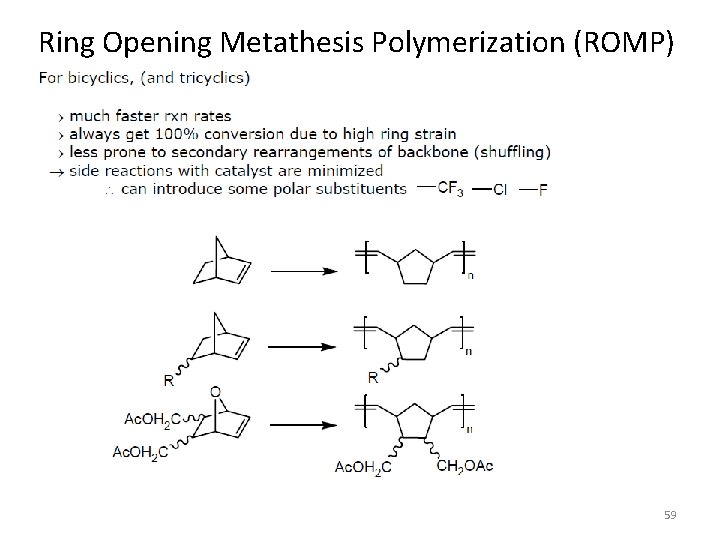
Ring Opening Metathesis Polymerization (ROMP) 59

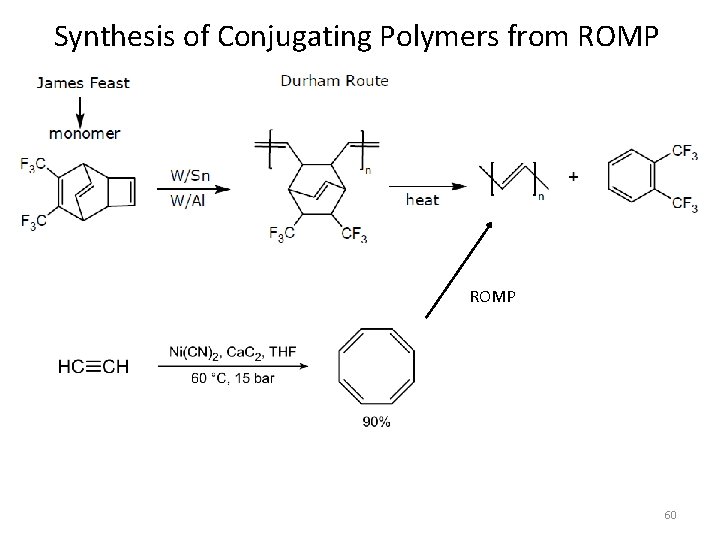
Synthesis of Conjugating Polymers from ROMP 60

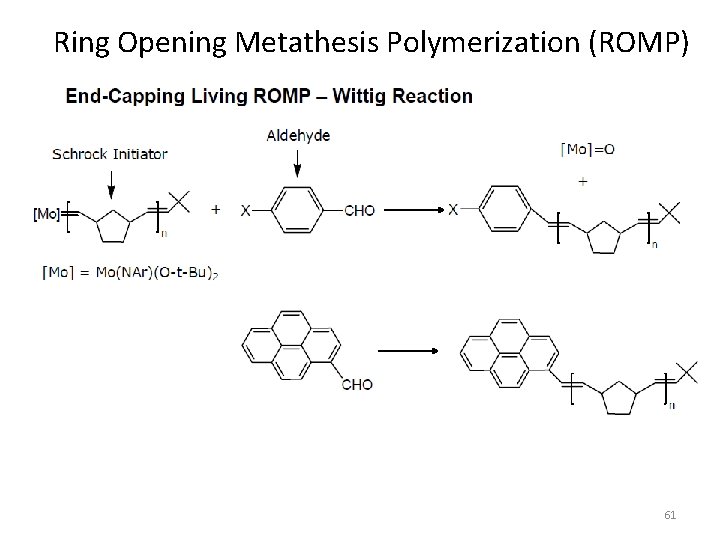
Ring Opening Metathesis Polymerization (ROMP) 61

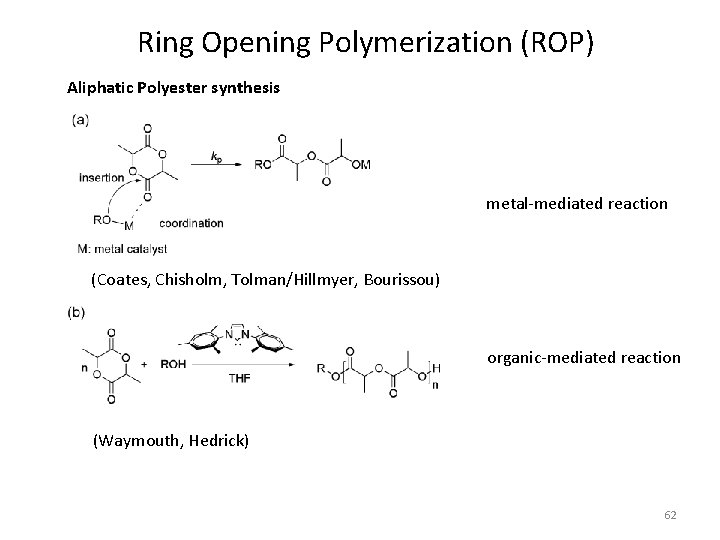
Ring Opening Polymerization (ROP) Aliphatic Polyester synthesis metal-mediated reaction (Coates, Chisholm, Tolman/Hillmyer, Bourissou) organic-mediated reaction (Waymouth, Hedrick) 62

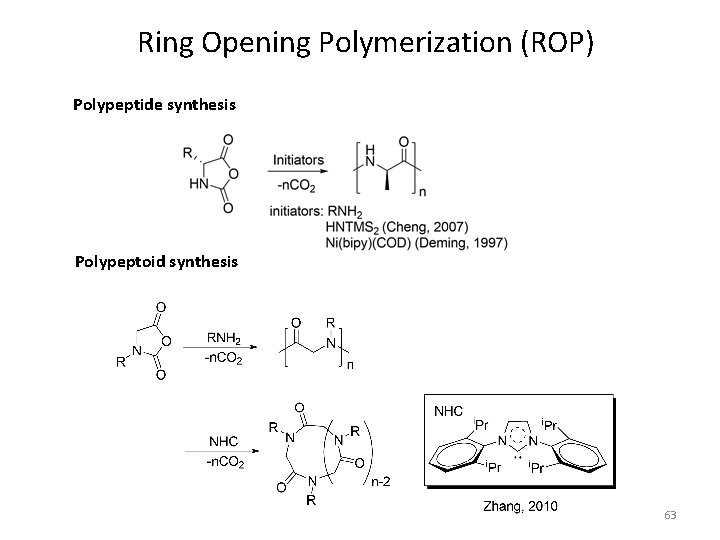
Ring Opening Polymerization (ROP) Polypeptide synthesis Polypeptoid synthesis 63

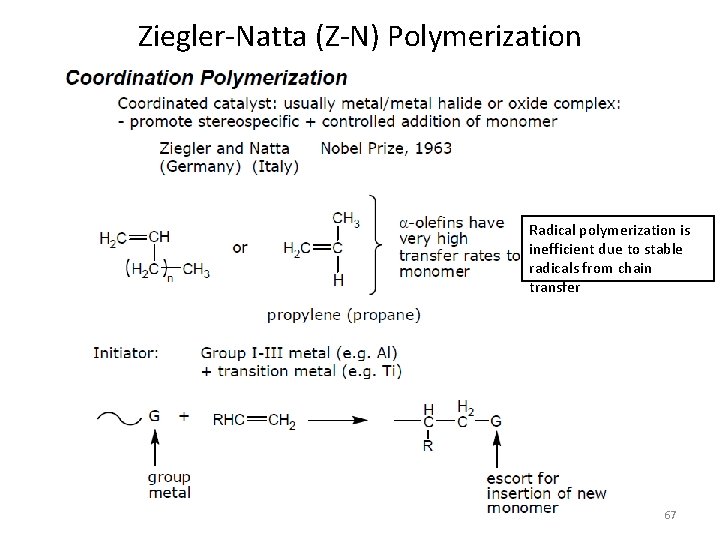
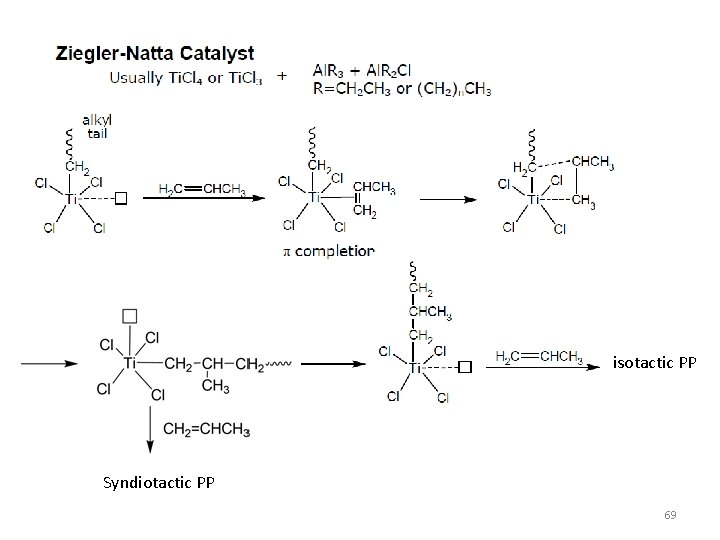
Coordination Polymerization Ziegler-Natta Polymerization (50 -60’s) • • Stereochemical Control Polydisperse products Statistical Compositions and Sequences Limited set of useful monomers, i. e. olefins • SINGLE SITE CATALYSTS 64

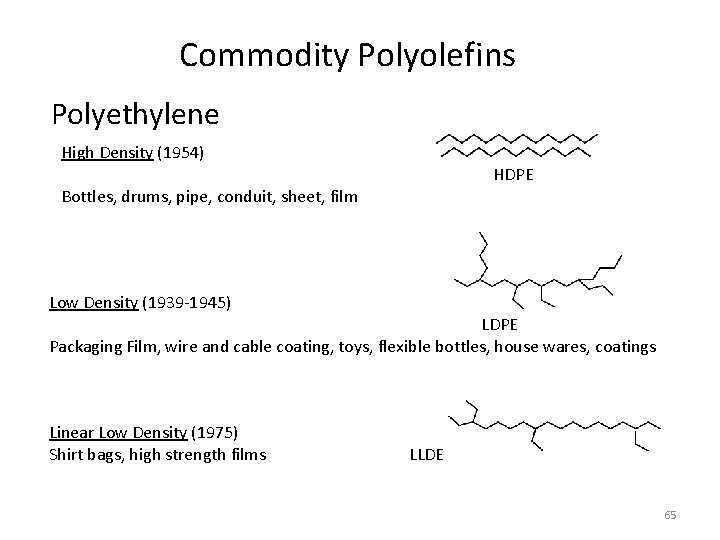
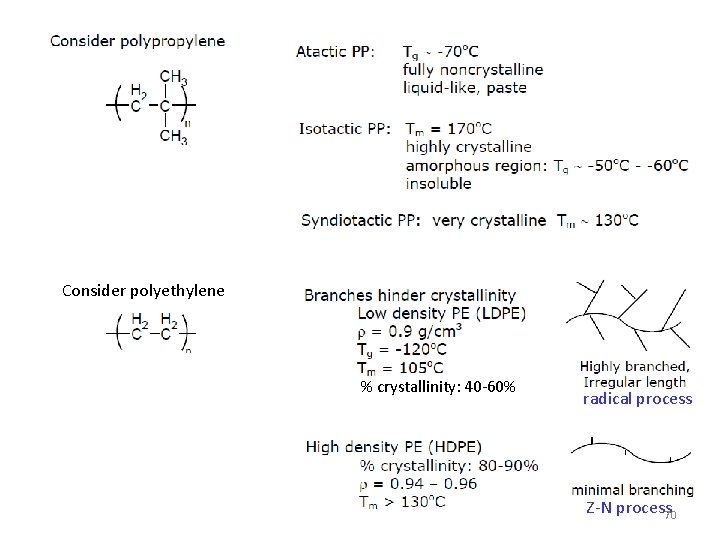
Commodity Polyolefins Polyethylene High Density (1954) HDPE Bottles, drums, pipe, conduit, sheet, film Low Density (1939 -1945) LDPE Packaging Film, wire and cable coating, toys, flexible bottles, house wares, coatings Linear Low Density (1975) Shirt bags, high strength films LLDE 65

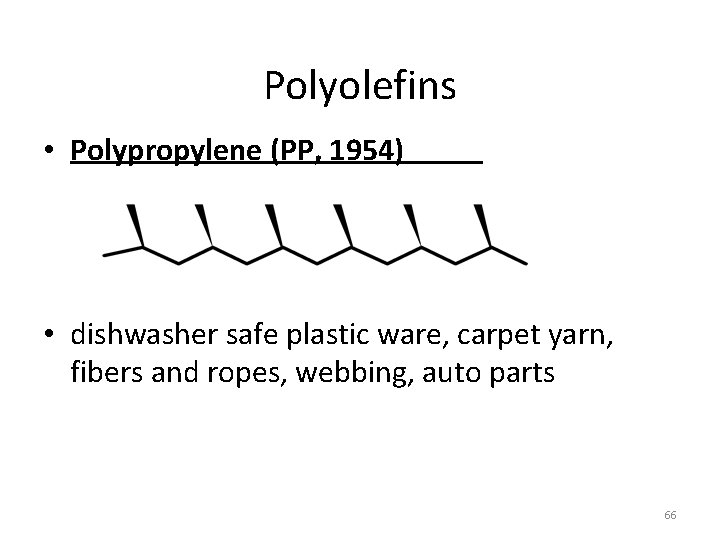
Polyolefins • Polypropylene (PP, 1954) • dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts 66

Ziegler-Natta (Z-N) Polymerization Radical polymerization is inefficient due to stable radicals from chain transfer 67

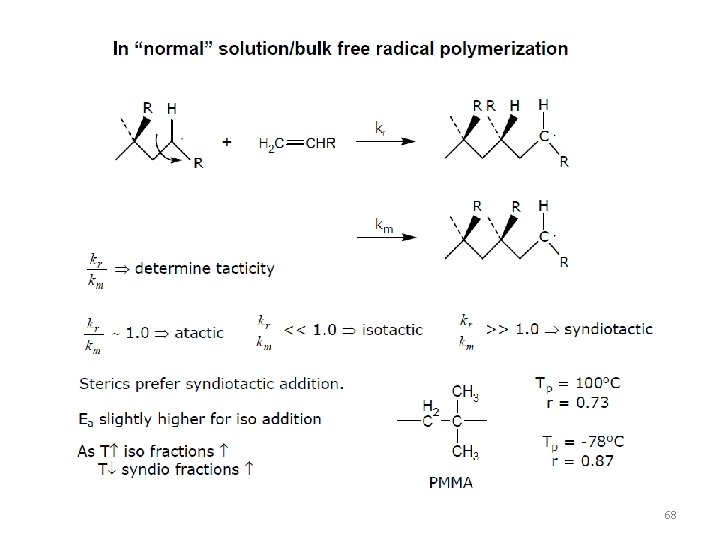
68

isotactic PP Syndiotactic PP 69

Consider polyethylene % crystallinity: 40 -60% radical process Z-N process 70

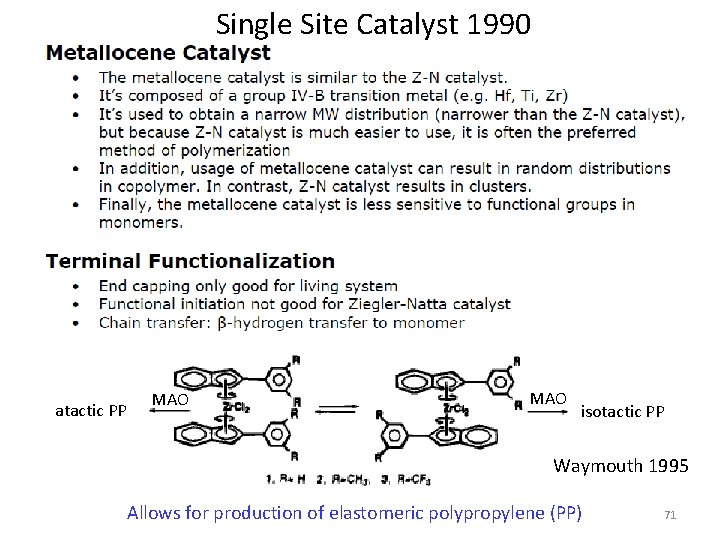
Single Site Catalyst 1990 atactic PP MAO isotactic PP Waymouth 1995 Allows for production of elastomeric polypropylene (PP) 71

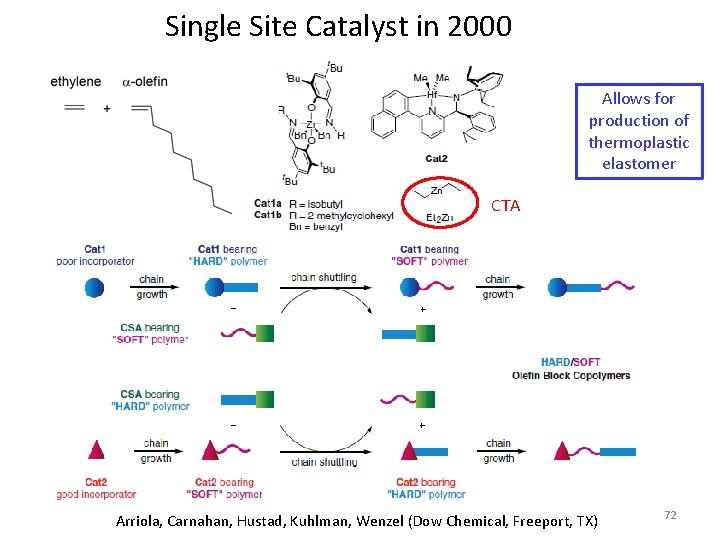
Single Site Catalyst in 2000 Allows for production of thermoplastic elastomer CTA Arriola, Carnahan, Hustad, Kuhlman, Wenzel (Dow Chemical, Freeport, TX) 72

Acknowledgement • MIT Open. Course. Ware: Synthesis of Polymers by Dr. Paula Hammond http: //ocw. mit. edu/courses/chemical-engineering/10 -569 -synthesis-of-polymers-fall-2006/lecture-notes/ • Note by USM Dr. Daniel Savin and Dr. Derek Patton http: //www. usm. edu/polymerkinetics/ • Note by LSU Dr. Daly • Polymer Chemistry, 2 nd edition, Hiemenz and Lodge • Principle of Polymerization, 4 th edition, Odian • Polymer Chemistry, 4 th edition, Pan, Zheijiang University • “Functional Polymers via Anionic Polymerizations. ” Akira Hirao, 1997 ACS Symposium Series. • “New Polymer Synthesis by Nitroxide Mediated Living Radical Polymerizations. ” Craig Hawker, 2001, Chem. Rev. • “Atom Transfer Radical Polymerization. ” Krzysztof Matyjaszewski, 2001, Chem. Rev. • “Copper(I)-Catalyzed Atom Transfer Radical Polymerization. ” Krzysztof Matyjaszewski and Timothy Patten, 1999, Acc. Chem. Res. • “Toward Living Radical Polymerization. ” Graeme Moad, Ezio Rizzardo, San Thang, 2008, Acc. Chem. Res. • “Living Radical Polymerization by the RAFT Process. ” Graeme Moad, Ezio Rizzardo, San Thang, 2005, Aust. J. Chem. • “Living ring-opening metathesis polymerization catalyzed by well-characterized transition-metal alkylidene complexes. ” Richard Schrock, 1990, Acc. Chem. Res. • “The Development of L 2 X 2 Ru. CHR Olefin Metathesis Catalysts: An Organometallic Success Story. ” Robert Grubbs, 2001, Acc. Chem. Soc. • “Organocatalytic Ring-Opening Polymerization. ” Robert Waymouth, James Hedrick, 2007, Chem. Rev. • “Controlled Ring-Opening Polymerization of Lactide and Glycolide. ” Didier Bourissou, 2004, Chem. Rev. • “Synthesis of Well-Defined Polypeptide-Based Materials via the Ring-Opening Polymerization of α-Amino Acid NCarboxyanhydrides. ” Nikos Hadjichristidis, 2009, Chem. Rev. 73