Polymer and Polymerization Prepared by Dental Materials Department
Polymer and Polymerization Prepared by: Dental Materials Department Yenepoya Dental College Yenepoya University, Mangalore. 1

Polymers in Dentistry • • • Dentures (base, liner, & artificial teeth Rubber impression materials Gloves Cements Sealants etc. 2
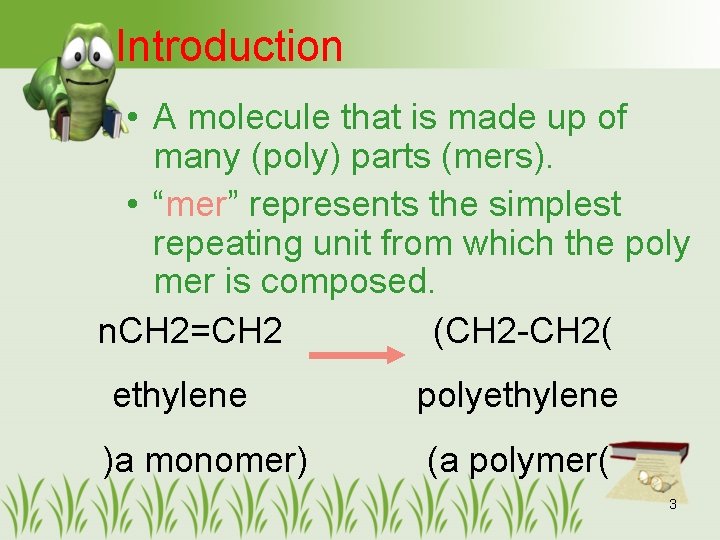
Introduction • A molecule that is made up of many (poly) parts (mers). • “mer” represents the simplest repeating unit from which the poly mer is composed. n. CH 2=CH 2 (CH 2 -CH 2( ethylene polyethylene )a monomer) (a polymer( 3
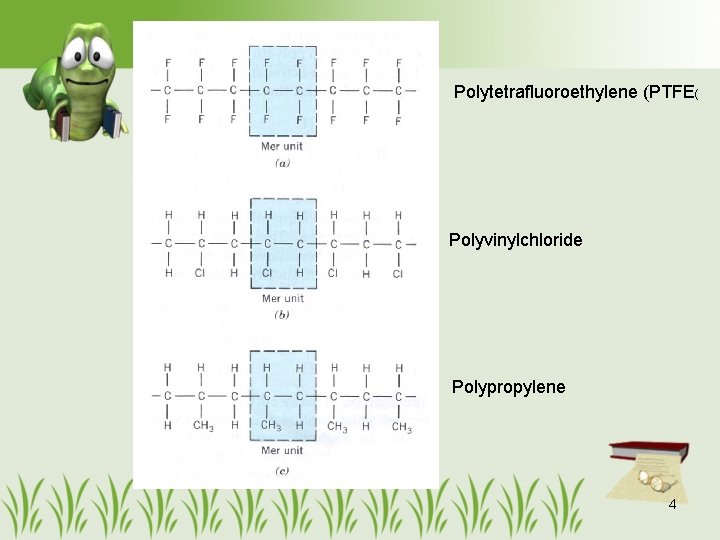
Polytetrafluoroethylene (PTFE( Polyvinylchloride Polypropylene 4
Introduction • Long-chain molecules formed by covalent bonding – monomer+… • Homopolymer, Copolymer, Terpolymer, … • The chains are held together either by secondary bonding forces or by primary covalent bonding forces through crosslinks between chains. • Natural or Synthetic 5
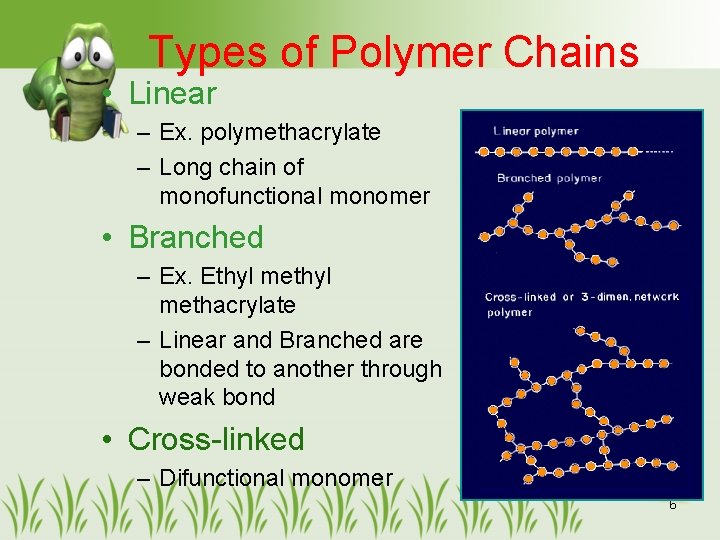
Types of Polymer Chains • Linear – Ex. polymethacrylate – Long chain of monofunctional monomer • Branched – Ex. Ethyl methacrylate – Linear and Branched are bonded to another through weak bond • Cross-linked – Difunctional monomer 6
Types of Polymer Chains • Cross-linked – Connected between chains – 3 -D or network polymers – More viscous, better physical properties – Min. x-linked ==> good elasticity, rubbery – Max. x-linked ==> stiff, stable – Insoluble in organic solvent 7
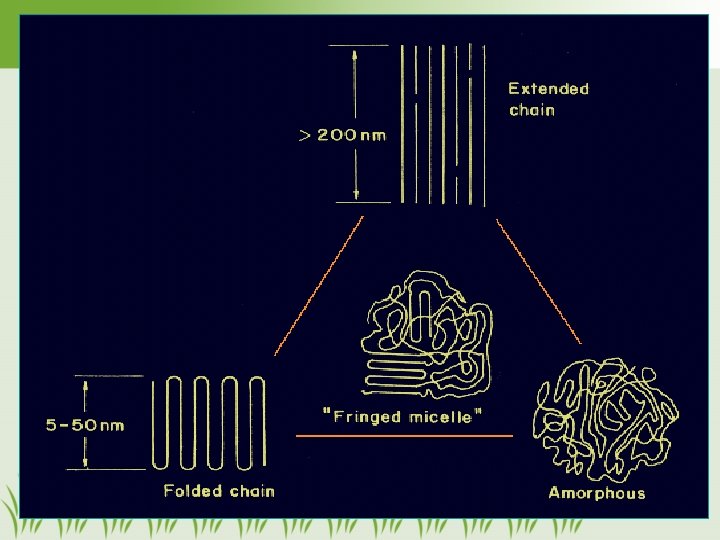
Polymer Structures • Amorphous polymers – Randomly coiled like spaghetti – Translucent or transparent because of the lack of crystallinity – Tend to be flexible rather than brittle – ex. elastomeric impression materials 8
Polymer Structures (con’t) • Crystalline polymers – Only portion of the polymer are crystalline (semi-crystalline). – Linear > cross-linked or branched polymers – Increase tensile strength but also increase brittleness and opacity 9
10
Classifications • Type of repeating unit – Homopolymer – Copolymer • Thermal processing behavior – Thermoplastics – Thermosets 11
Classifications • Polymerization mechanism – Condenstion/ Step polymerization – Addition/ Chain polymerization 12
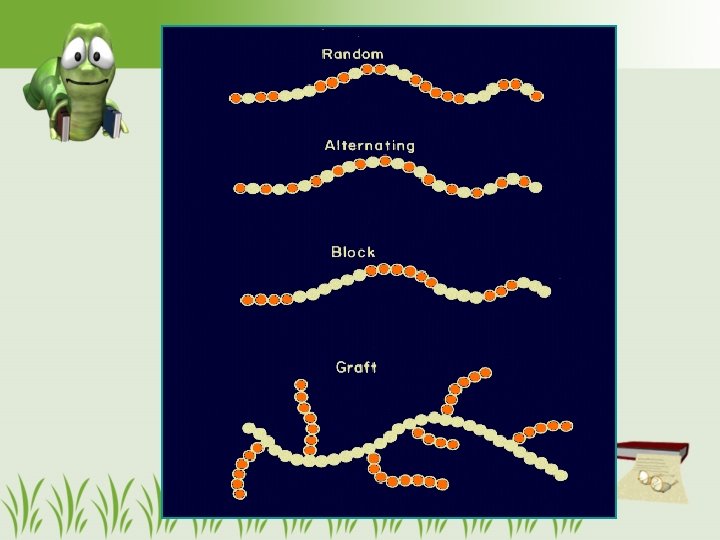
Type of repeating unit • Homopolymer – has mer units of the same type • Copolymer – 2 or more mer units – random coplolymer – alternating copolymer – block copolymer – graft copolymer 13
Thermal Processing Behavior • Thermal properties • Tg (glass-transition temperator) • Remember secondary bonds? – Van der Waals, Hydrogen • Above Tg, the secondary bonds between chains are broken. The polymer chains can move more freely. 15
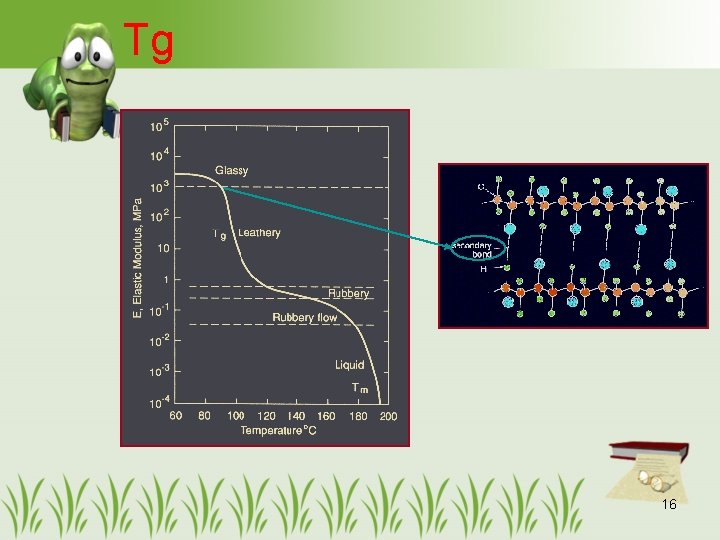
Tg 16

Tg (con’t) • Same polymers – Higher MW, higher Tg • Increased MW becomes less important when the chain length approaches the critical length. • At this length, the dislodging force breaks the cohesive bond of the chain instead of the polar bonds between chains. 17
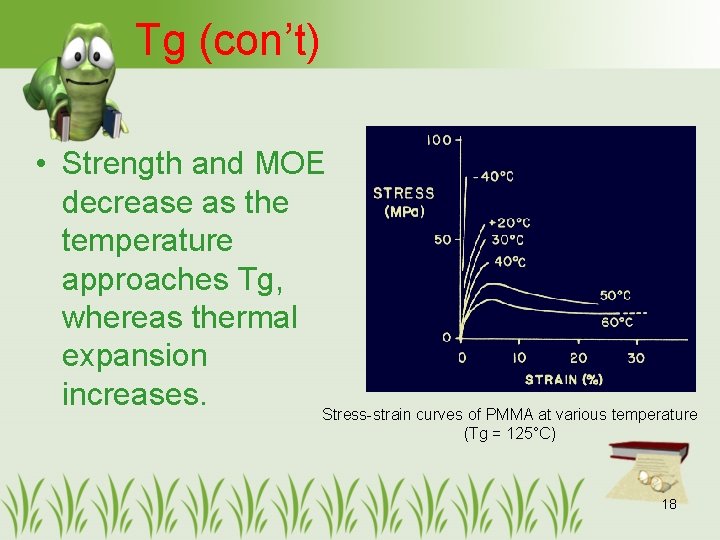
Tg (con’t) • Strength and MOE decrease as the temperature approaches Tg, whereas thermal expansion increases. Stress-strain curves of PMMA at various temperature (Tg = 125°C) 18

Thermal Processing Behavior • Thermoplastic materials – Soften when heated/reheated above their Tg. – Can be shaped and will harden on cooling. – Usually soluble in organic solvents, fusible – Better flexural and impact properties – ex. polyolefins (poly ethylene, polypropylene), polyvinyl chloride, impression compounds, acrylics 19
Thermal Processing Behavior • Thermosetting materials – Do not soften on reheating – Usually cross-linked, insoluble and infusible – Superior abrasion resistance and dimensional stability – ex. epoxy, phenol-formaldehyde 20
Polymerization Mechanisms • Condensation/ Step Polymerization – Step reaction between function groups of monomers – Monomer + Monomer – Monomer dimer trimer tetramer … – Chain grows slowly. • Addition/ Chain Polymerization – Initiator, Free radical – Monomer + Free radical – Chain gets longer. (propagation) – Stopped when free radicals are eliminated. (termination) 21
Condensation or Step Polymerization 1. Polymerization is accompanied by repeated elimination of small molecules or 2. Functional groups are repeated in the polymer chain • The term “step” is preferred because the repeating units can be joined by functional groups without the formation of a by-product e. g. in polyurethane. 22
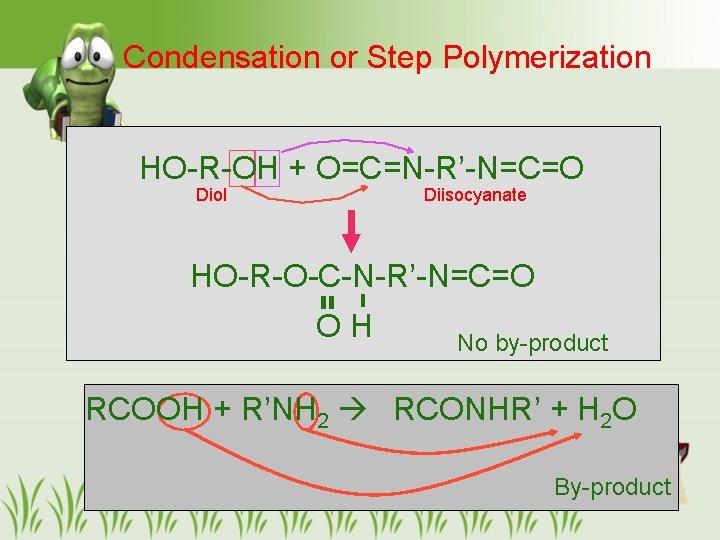
Condensation or Step Polymerization HO-R-OH + O=C=N-R’-N=C=O Diol Diisocyanate HO-R-O-C-N-R’-N=C=O OH No by-product RCOOH + R’NH 2 RCONHR’ + H 2 O By-product 23

Condensation or Step Polymerization • Slow, tend to stop before the molecules have reached a truly great size. • Examples: – Polysulfide: by-product = water – Condensation silicone: by-product = alcohol – Collagen, DNA, RNA formations • By-products affect the dimensional stability when evaporate. 24
Additional or Chain Polymerization • No change in composition • Monomer and polymer have the same empirical formula. • Can easily produce giant molecules of almost unlimited size • Needs an unsaturated group; i. e. a double bond • Exothermal reaction 25
Stage in chain polymerization • Induction • Propagation • Termination 26

Induction • Activation & Initiation • Free radical: an atom or group of atoms possessing an odd (unpaired) electron generated by activation of radical-producing molecules (initiator) with light, chemical, heat or energy transfer from another compound 27
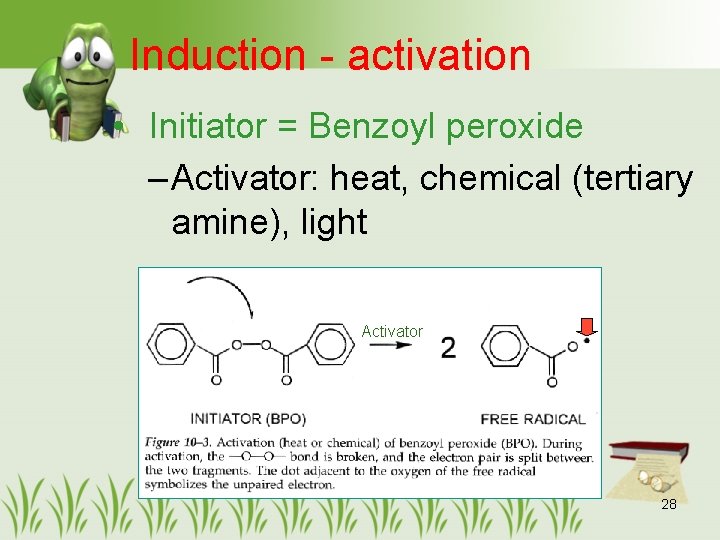
Induction - activation • Initiator = Benzoyl peroxide – Activator: heat, chemical (tertiary amine), light Activator 28
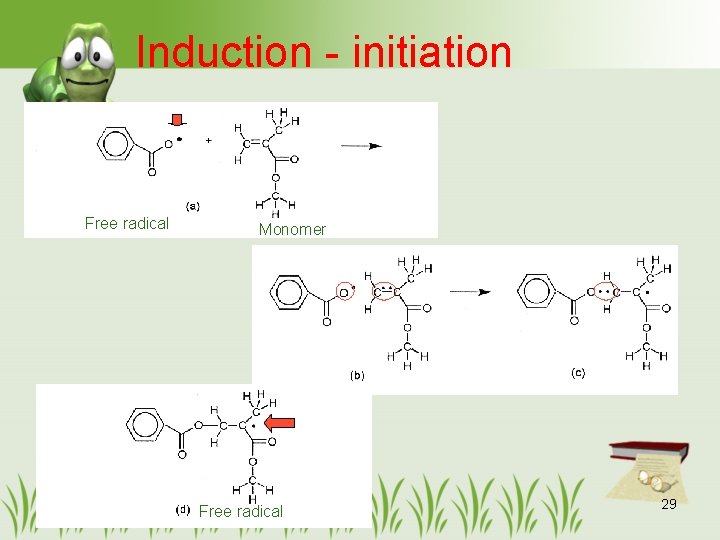
Induction - initiation Free radical Monomer Free radical 29
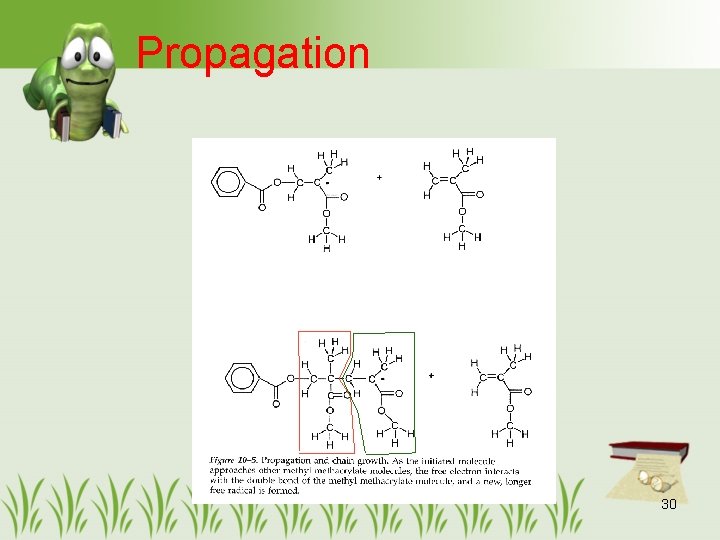
Propagation 30
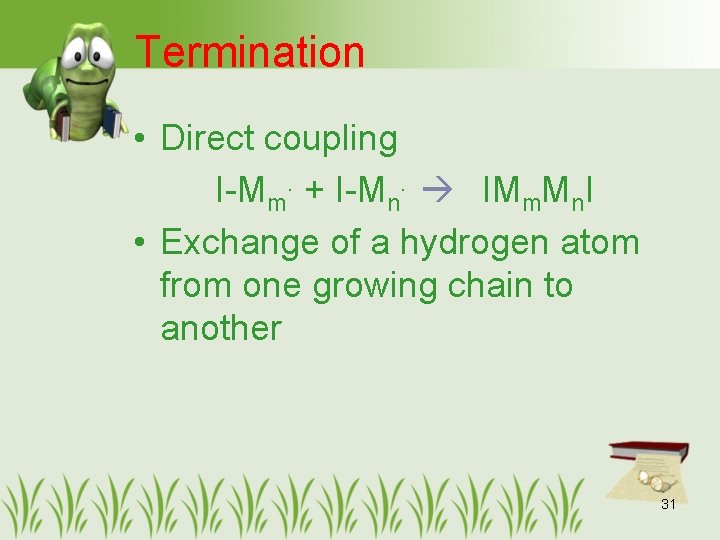
Termination • Direct coupling I-Mm. + I-Mn. IMm. Mn. I • Exchange of a hydrogen atom from one growing chain to another 31
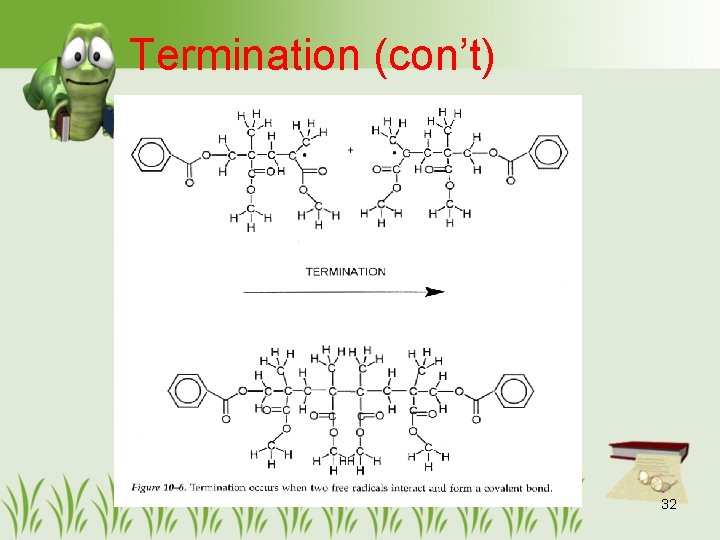
Termination (con’t) 32
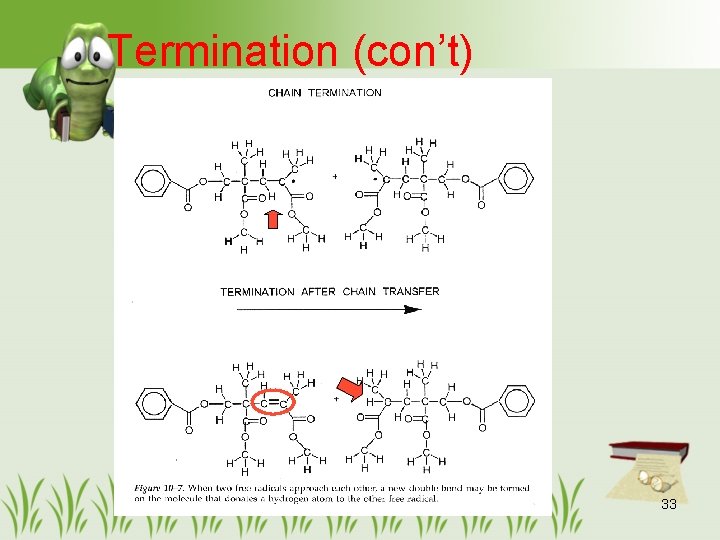
Termination (con’t) 33
Degree of Polymerization (DP( • Degree of conversion • The degree to which monomers convert into a polymer • Average number of mers or repeating units per polymer molecules • Each chain may have a small or large number of mers and the length of each chain may be different. • Normally, there were no 100% DP 34
Inhibition of Polymerization • Impurities in the monomer often inhibit polymerization. – Impurities ~ free radicals – Influences the length of the initiation period and DP – Retards the polymerization reaction – e. g. hydroquinone, O 2 35
Modifying Polymers • Copolymerization • Cross-linking • Plasticizing 36
Copolymerization • Two or more chemically different monomers “copolymer” • Random, Block, Graft • Block and graft polymers often show improved impact strength. • ex. ethyl acrylate + MMA improves flexibility of denture plastic 37
Cross-linking • Induce covalent bonding between polymer chains • Increases strength • Decreases solubility and sorption • ex. denture teeth 38

Plasticizing • Reduce the softening or fusion temperatures • Strength, hardness and brittleness • Types of plasticizers – External plasticizer – Internal plasticizer 39

Plasticizers • External plasticizer – It penetrates between the macromolecules and increases the intermolecular spacing. – Acts to partially neutralize secondary bonding between chains. – Since they do not covalently bond to the polymer, they eventually leach out. – ex. resilient denture liners = acrylic polymers plasticized with dibutyl phthalate 40
Plasticizers (con’t) • Internal plasticizer – Same as copolymerization – The larger monomer molecules increase intermolecular spacing. – ex. butyl acrylate + MMA 41

Factors to control properties • • • Chemical composition Molecular weight Chain structure Crystallinity Copolymerization Blending 42
Molecular Weight • Molecular weight (MW) of polymer molecule = MW of the various mers multiplied by the number of the mers. • May range from thousands to millions of MW units • The higher the MW of the polymer made from a single monomer, the higher the degree of polymerization (DP). 43
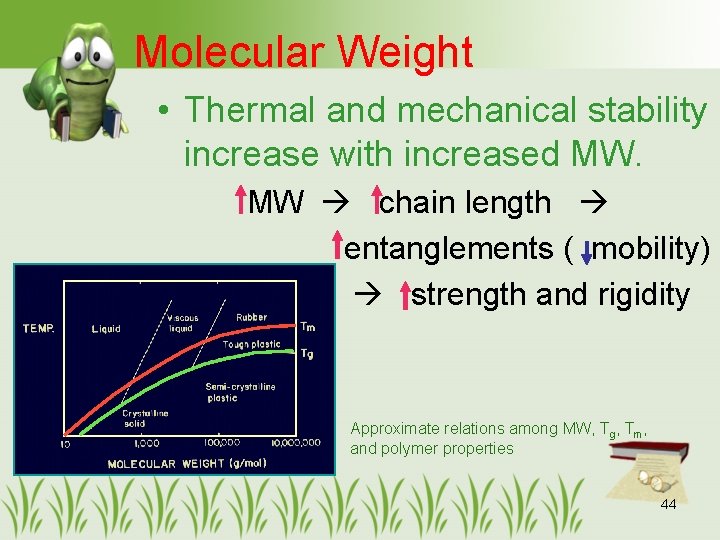
Molecular Weight • Thermal and mechanical stability increase with increased MW. MW chain length entanglements ( mobility) strength and rigidity Approximate relations among MW, Tg, Tm, and polymer properties 44
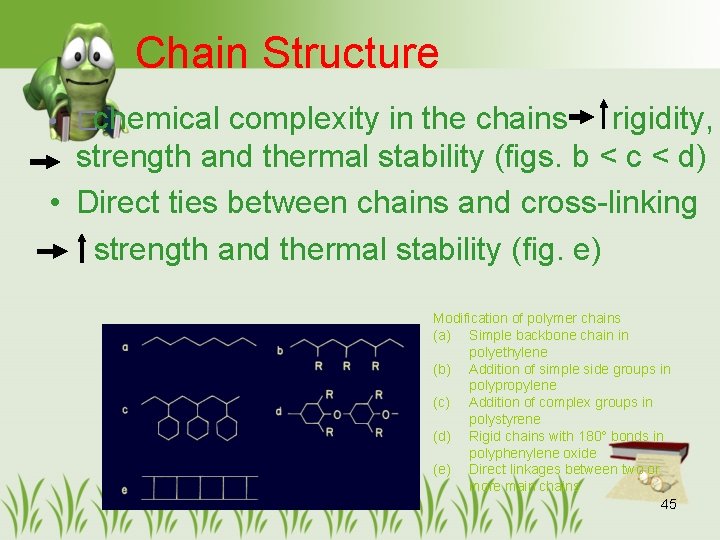
Chain Structure • �chemical complexity in the chains rigidity, strength and thermal stability (figs. b < c < d) • Direct ties between chains and cross-linking strength and thermal stability (fig. e) Modification of polymer chains (a) Simple backbone chain in polyethylene (b) Addition of simple side groups in polypropylene (c) Addition of complex groups in polystyrene (d) Rigid chains with 180° bonds in polyphenylene oxide (e) Direct linkages between two or more main chains 45

Crystallinity • Crystallinity in semicrystalline polymer e. g. Polyethylene and Polyamide (nylon) strength and thermal stability. 46

Blending • Blending of polymers with fillers can increase strength if the fillers are stronger than the matrix polymer and have good adhesion between them. 47
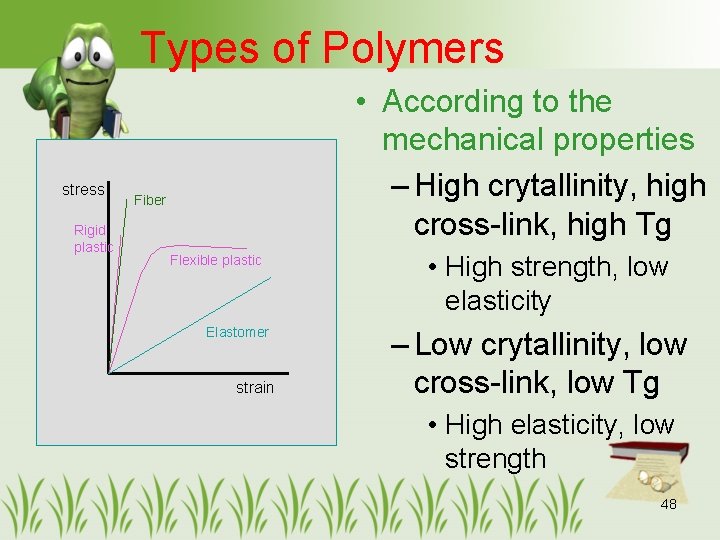
Types of Polymers stress Rigid plastic • According to the mechanical properties – High crytallinity, high cross-link, high Tg Fiber Flexible plastic Elastomer strain • High strength, low elasticity – Low crytallinity, low cross-link, low Tg • High elasticity, low strength 48
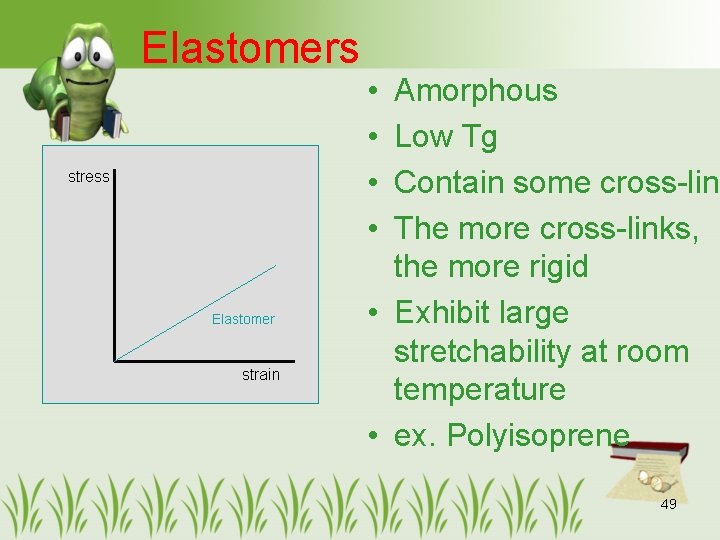
Elastomers stress Elastomer strain • • Amorphous Low Tg Contain some cross-link The more cross-links, the more rigid • Exhibit large stretchability at room temperature • ex. Polyisoprene 49
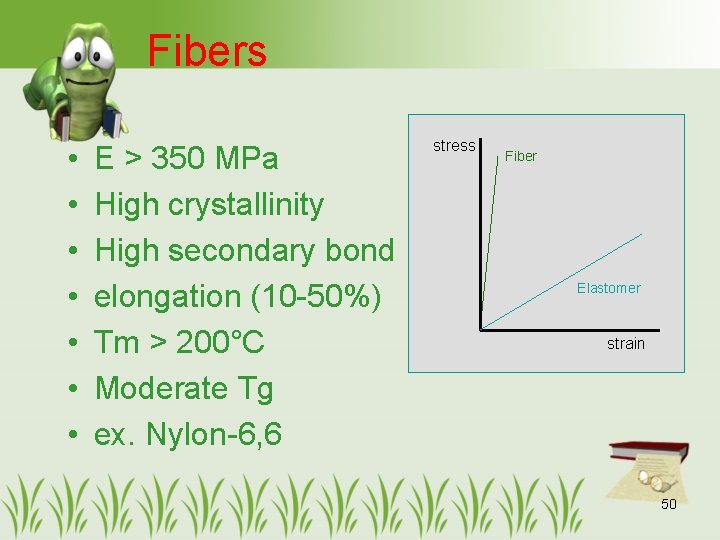
Fibers • • E > 350 MPa High crystallinity High secondary bond elongation (10 -50%) Tm > 200°C Moderate Tg ex. Nylon-6, 6 stress Fiber Elastomer strain 50
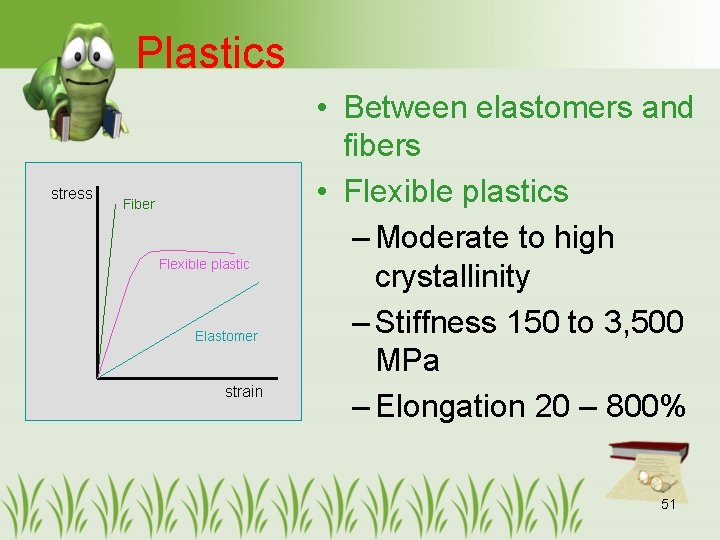
Plastics stress Fiber Flexible plastic Elastomer strain • Between elastomers and fibers • Flexible plastics – Moderate to high crystallinity – Stiffness 150 to 3, 500 MPa – Elongation 20 – 800% 51
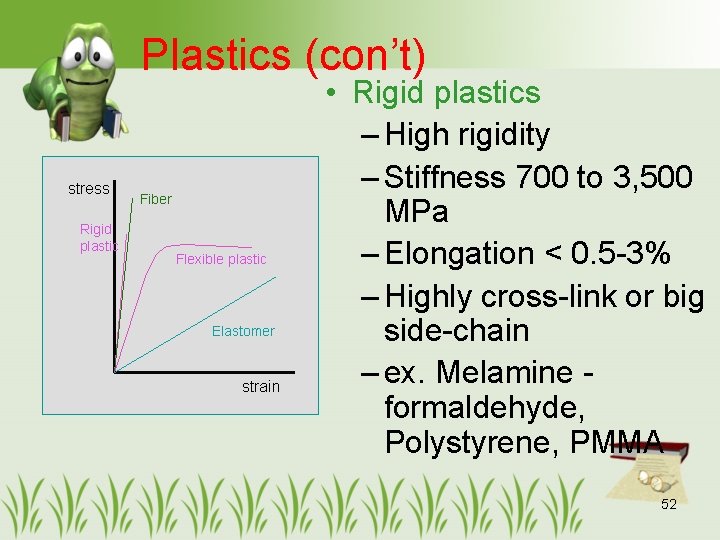
Plastics (con’t) stress Rigid plastic Fiber Flexible plastic Elastomer strain • Rigid plastics – High rigidity – Stiffness 700 to 3, 500 MPa – Elongation < 0. 5 -3% – Highly cross-link or big side-chain – ex. Melamine formaldehyde, Polystyrene, PMMA 52

Summary • • Structures Classifications Polymerization mechanisms Modifying polymers Molecular weight Factors to control properties Types of polymers 53
- Slides: 53