POLYFUNCTIONAL ORGANIC COMPOUND PRESENTED BY Prof Dr Suyano

POLYFUNCTIONAL ORGANIC COMPOUND PRESENTED BY Prof. Dr. Suyano, M. Si. DEPARTMENT OF CHEMISTRY STATE UNIVERSITY OF SURABAYA 2020
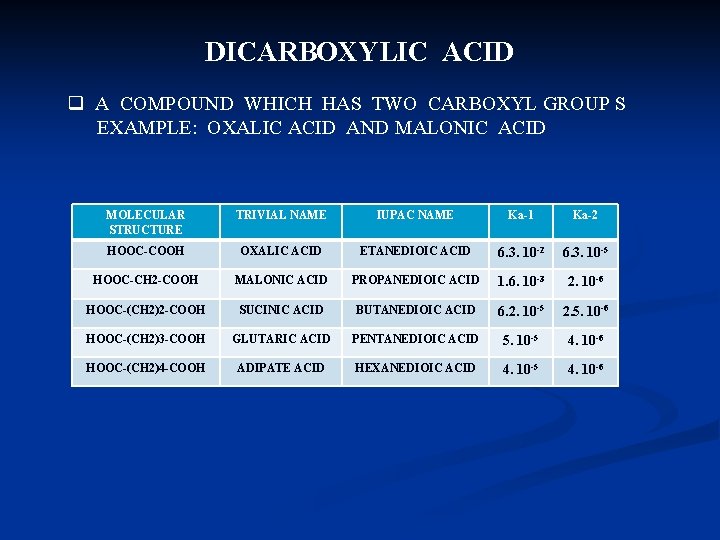
DICARBOXYLIC ACID q A COMPOUND WHICH HAS TWO CARBOXYL GROUP S EXAMPLE: OXALIC ACID AND MALONIC ACID MOLECULAR STRUCTURE TRIVIAL NAME IUPAC NAME Ka-1 Ka-2 HOOC-COOH OXALIC ACID ETANEDIOIC ACID 6. 3. 10 -2 6. 3. 10 -5 HOOC-CH 2 -COOH MALONIC ACID PROPANEDIOIC ACID 1. 6. 10 -3 2. 10 -6 HOOC-(CH 2)2 -COOH SUCINIC ACID BUTANEDIOIC ACID 6. 2. 10 -5 2. 5. 10 -6 HOOC-(CH 2)3 -COOH GLUTARIC ACID PENTANEDIOIC ACID 5. 10 -5 4. 10 -6 HOOC-(CH 2)4 -COOH ADIPATE ACID HEXANEDIOIC ACID 4. 10 -5 4. 10 -6
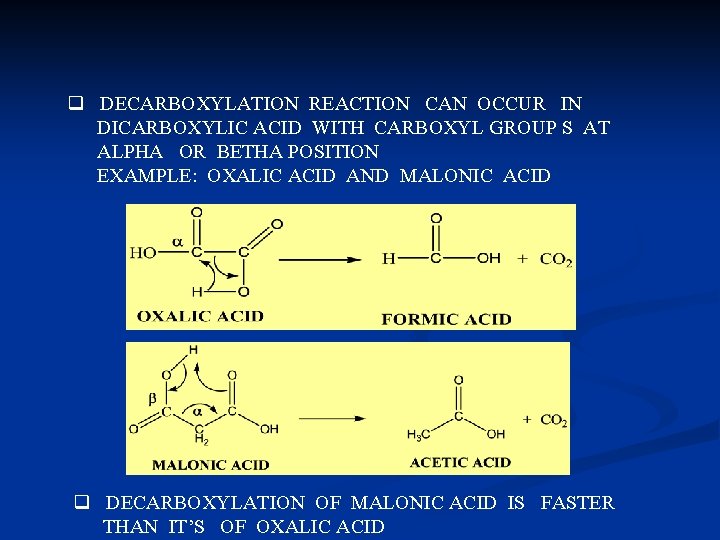
q DECARBOXYLATION REACTION CAN OCCUR IN DICARBOXYLIC ACID WITH CARBOXYL GROUP S AT ALPHA OR BETHA POSITION EXAMPLE: OXALIC ACID AND MALONIC ACID q DECARBOXYLATION OF MALONIC ACID IS FASTER THAN IT’S OF OXALIC ACID
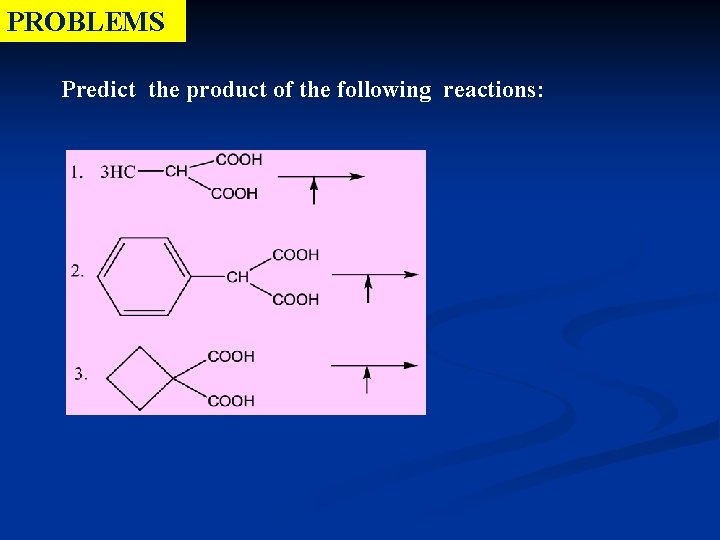
PROBLEMS Predict the product of the following reactions:
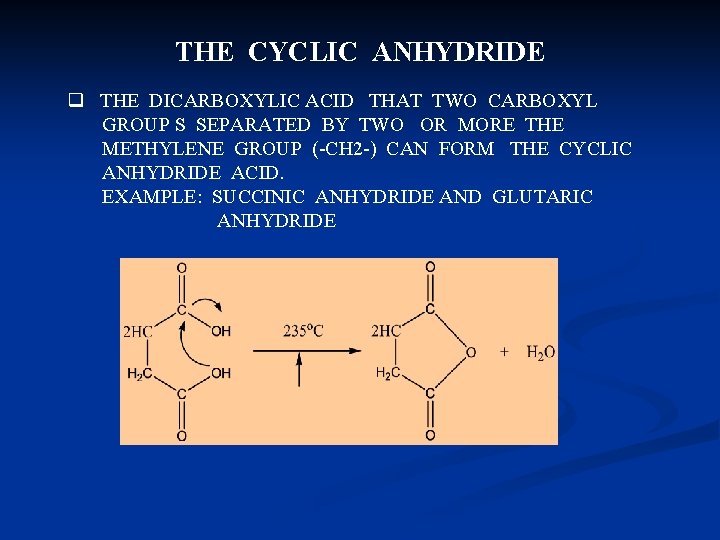
THE CYCLIC ANHYDRIDE q THE DICARBOXYLIC ACID THAT TWO CARBOXYL GROUP S SEPARATED BY TWO OR MORE THE METHYLENE GROUP (-CH 2 -) CAN FORM THE CYCLIC ANHYDRIDE ACID. EXAMPLE: SUCCINIC ANHYDRIDE AND GLUTARIC ANHYDRIDE
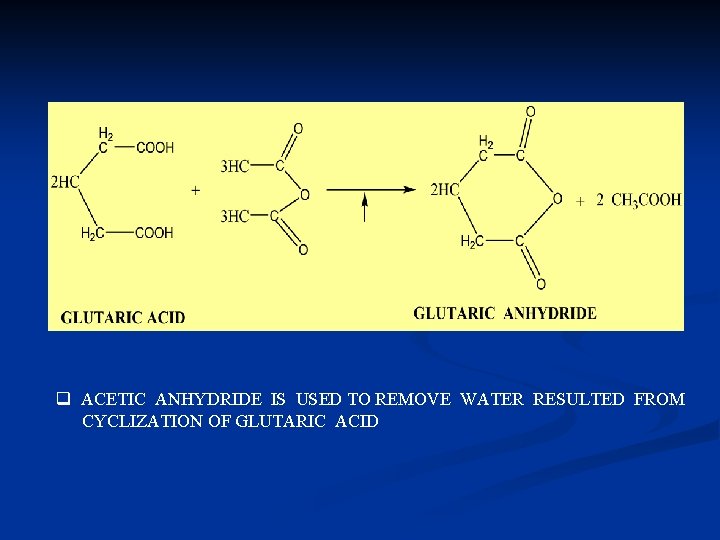
q ACETIC ANHYDRIDE IS USED TO REMOVE WATER RESULTED FROM CYCLIZATION OF GLUTARIC ACID
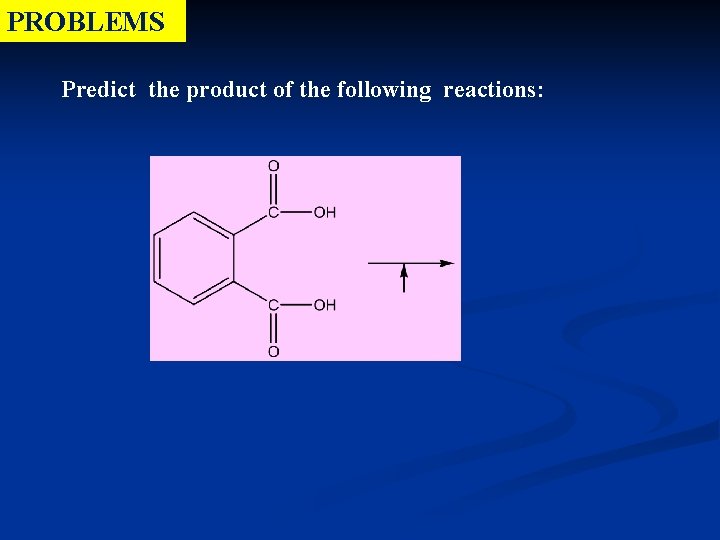
PROBLEMS Predict the product of the following reactions:
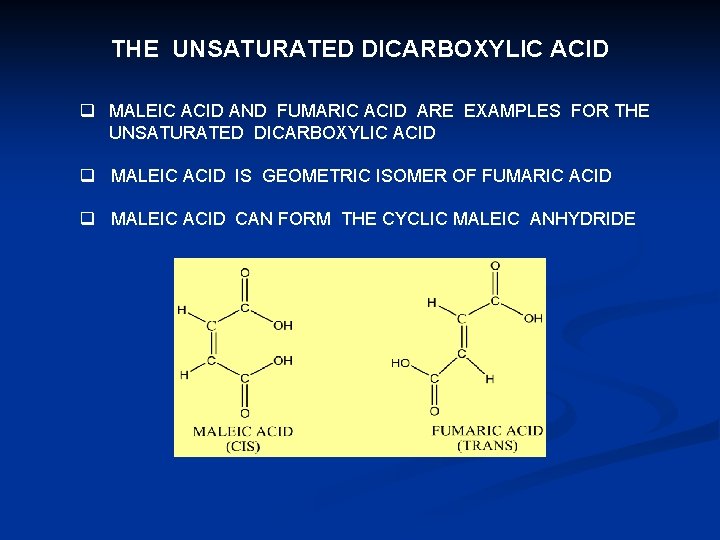
THE UNSATURATED DICARBOXYLIC ACID q MALEIC ACID AND FUMARIC ACID ARE EXAMPLES FOR THE UNSATURATED DICARBOXYLIC ACID q MALEIC ACID IS GEOMETRIC ISOMER OF FUMARIC ACID q MALEIC ACID CAN FORM THE CYCLIC MALEIC ANHYDRIDE
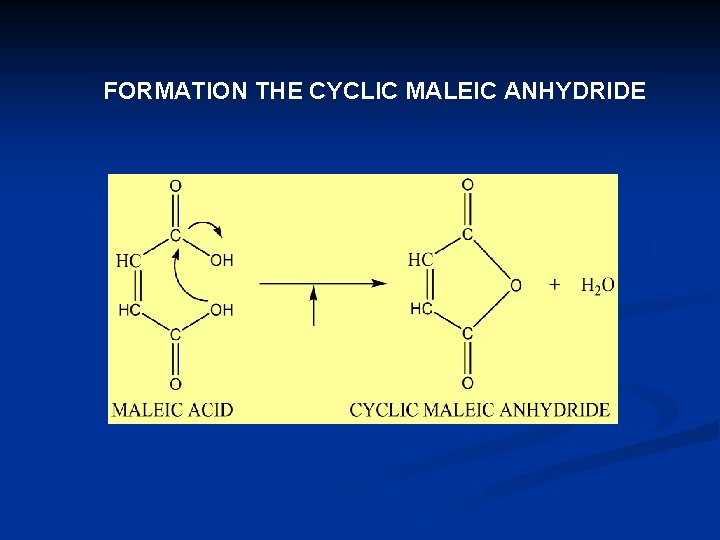
FORMATION THE CYCLIC MALEIC ANHYDRIDE
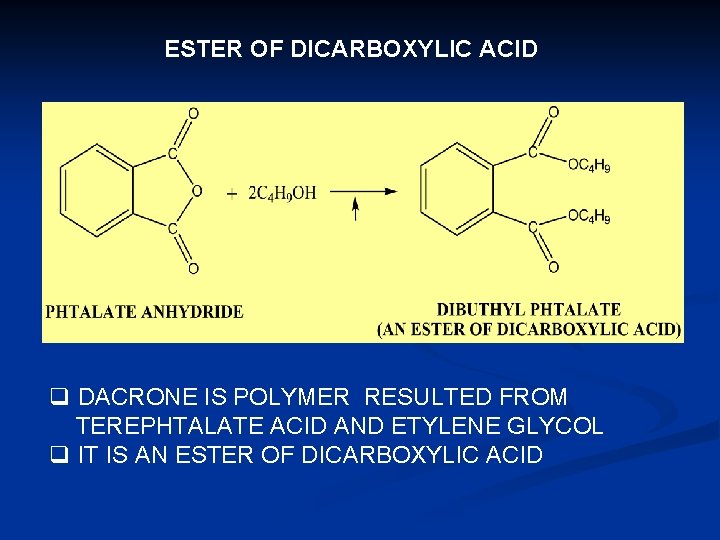
ESTER OF DICARBOXYLIC ACID q DACRONE IS POLYMER RESULTED FROM TEREPHTALATE ACID AND ETYLENE GLYCOL q IT IS AN ESTER OF DICARBOXYLIC ACID
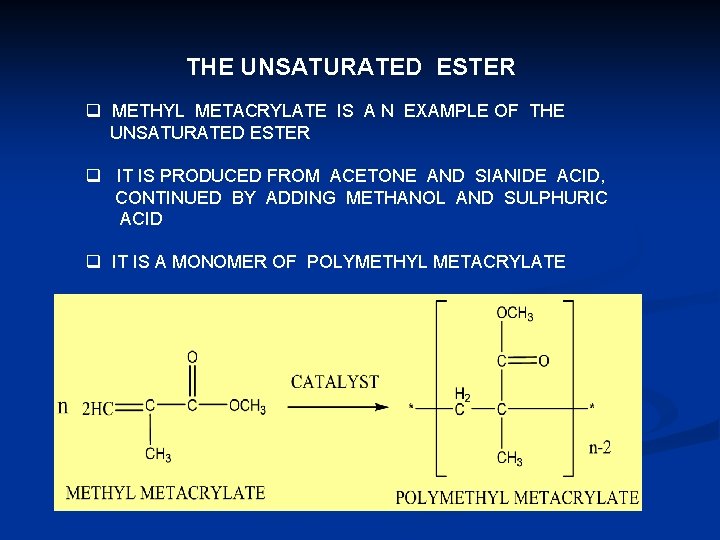
THE UNSATURATED ESTER q METHYL METACRYLATE IS A N EXAMPLE OF THE UNSATURATED ESTER q IT IS PRODUCED FROM ACETONE AND SIANIDE ACID, CONTINUED BY ADDING METHANOL AND SULPHURIC ACID q IT IS A MONOMER OF POLYMETHYL METACRYLATE

THE HYDROXY ACID q THE HYDROXY ACID IS THE CARBOXYLIC ACID CONTAINING THE HYDROXYL GROUP q THE FOLLOWING EXAMPLES ARE THE HYDROXY ACID
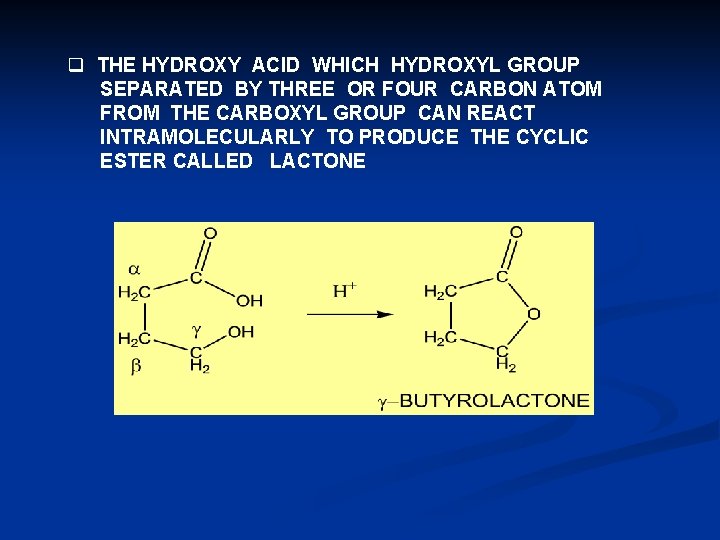
q THE HYDROXY ACID WHICH HYDROXYL GROUP SEPARATED BY THREE OR FOUR CARBON ATOM FROM THE CARBOXYL GROUP CAN REACT INTRAMOLECULARLY TO PRODUCE THE CYCLIC ESTER CALLED LACTONE
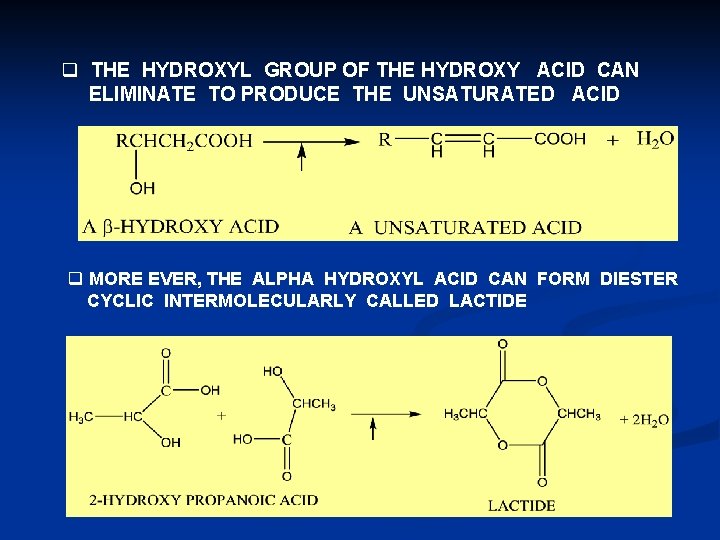
q THE HYDROXYL GROUP OF THE HYDROXY ACID CAN ELIMINATE TO PRODUCE THE UNSATURATED ACID q MORE EVER, THE ALPHA HYDROXYL ACID CAN FORM DIESTER CYCLIC INTERMOLECULARLY CALLED LACTIDE
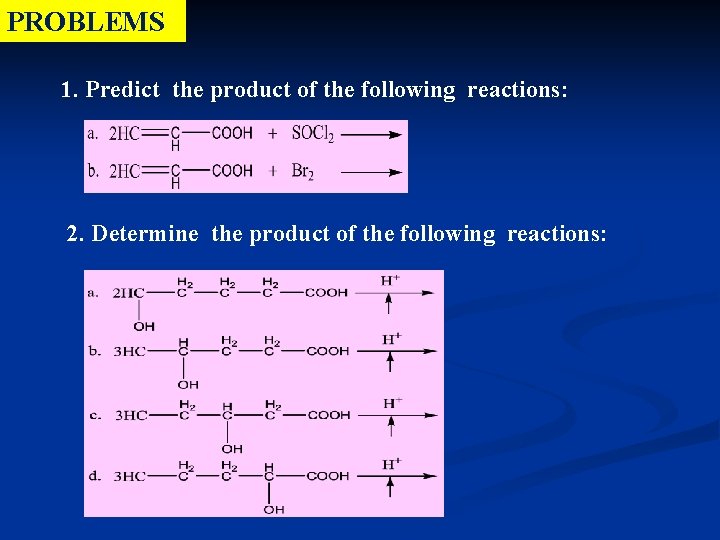
PROBLEMS 1. Predict the product of the following reactions: 2. Determine the product of the following reactions:
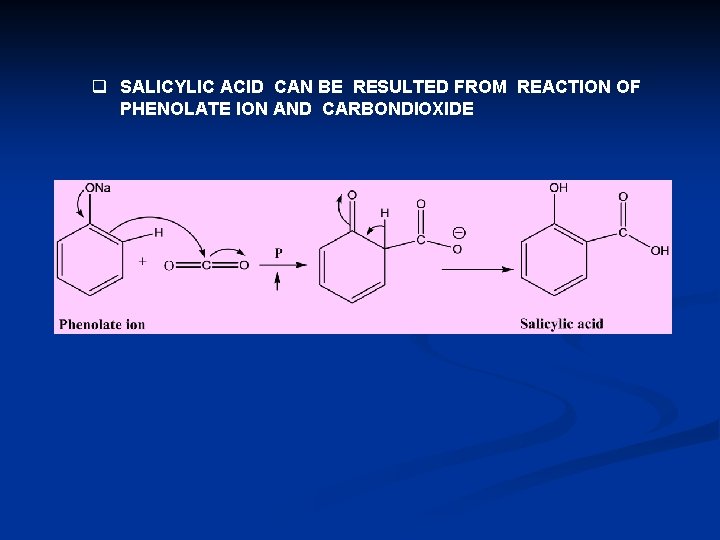
q SALICYLIC ACID CAN BE RESULTED FROM REACTION OF PHENOLATE ION AND CARBONDIOXIDE
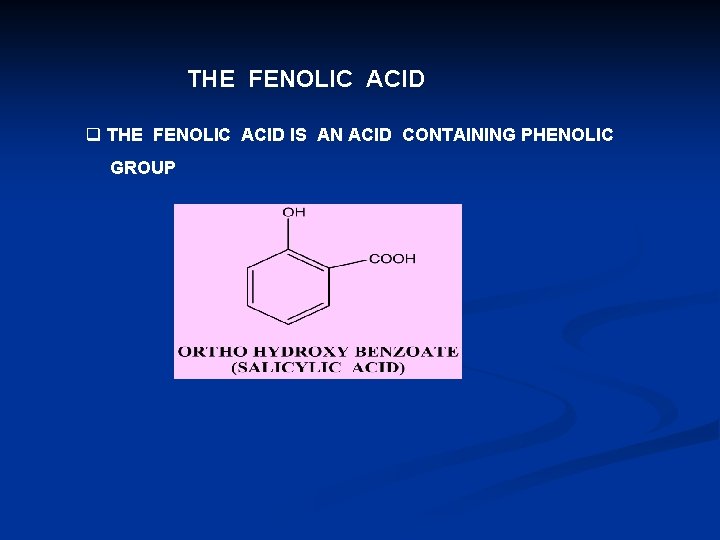
THE FENOLIC ACID q THE FENOLIC ACID IS AN ACID CONTAINING PHENOLIC GROUP
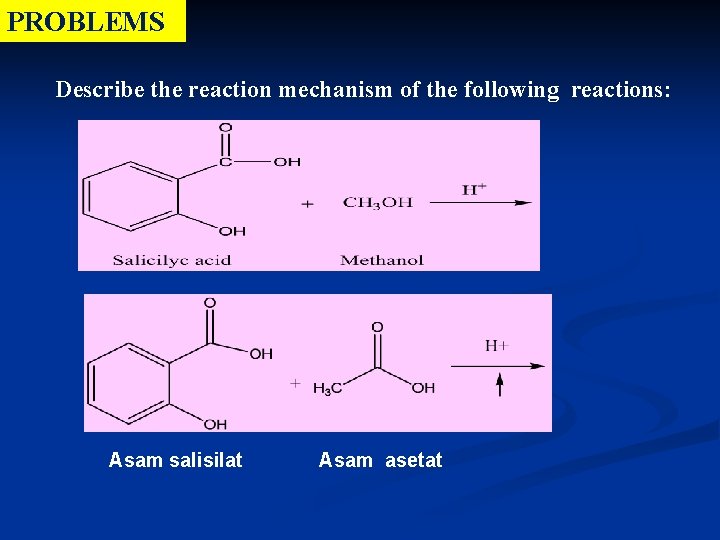
PROBLEMS Describe the reaction mechanism of the following reactions: Asam salisilat Asam asetat
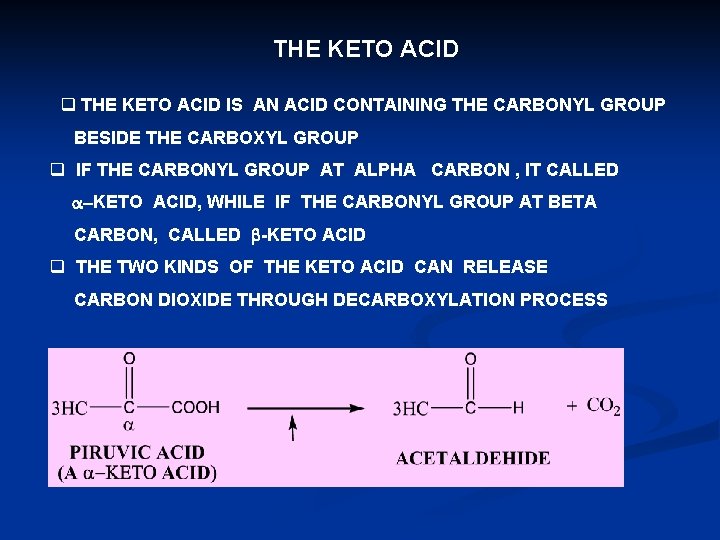
THE KETO ACID q THE KETO ACID IS AN ACID CONTAINING THE CARBONYL GROUP BESIDE THE CARBOXYL GROUP q IF THE CARBONYL GROUP AT ALPHA CARBON , IT CALLED a-KETO ACID, WHILE IF THE CARBONYL GROUP AT BETA CARBON, CALLED b-KETO ACID q THE TWO KINDS OF THE KETO ACID CAN RELEASE CARBON DIOXIDE THROUGH DECARBOXYLATION PROCESS
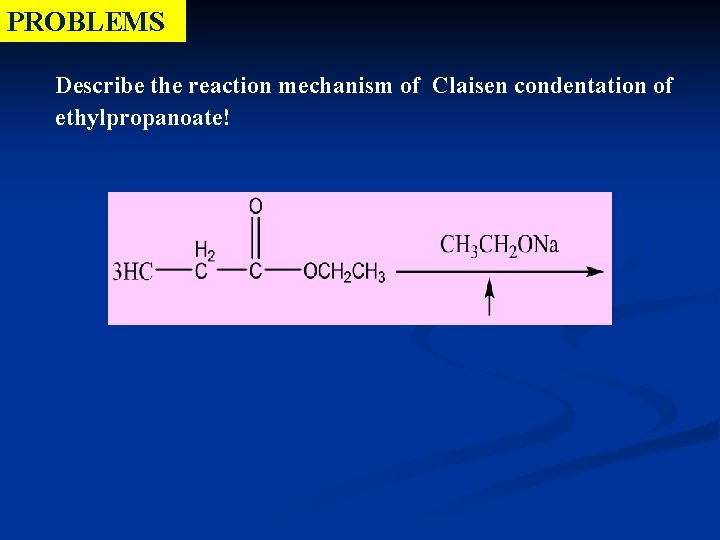
PROBLEMS Describe the reaction mechanism of Claisen condentation of ethylpropanoate!
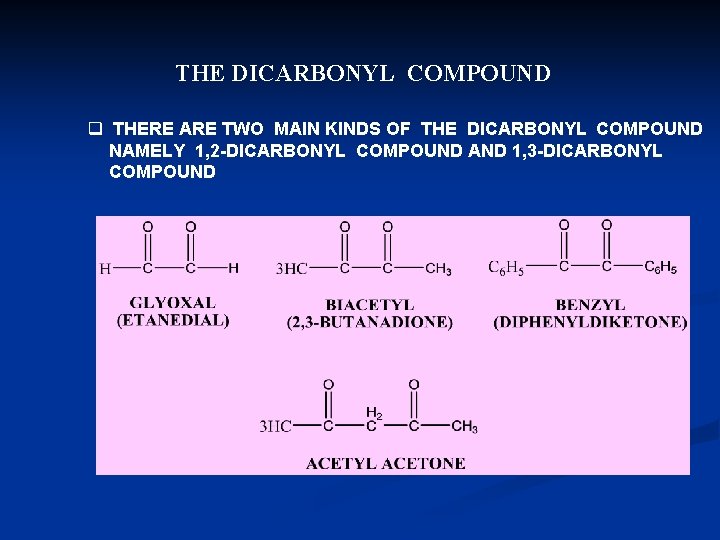
THE DICARBONYL COMPOUND q THERE ARE TWO MAIN KINDS OF THE DICARBONYL COMPOUND NAMELY 1, 2 -DICARBONYL COMPOUND AND 1, 3 -DICARBONYL COMPOUND
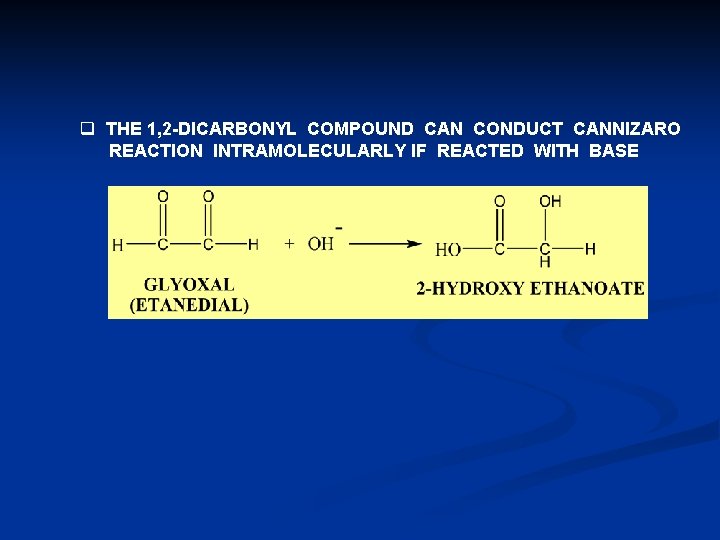
q THE 1, 2 -DICARBONYL COMPOUND CAN CONDUCT CANNIZARO REACTION INTRAMOLECULARLY IF REACTED WITH BASE
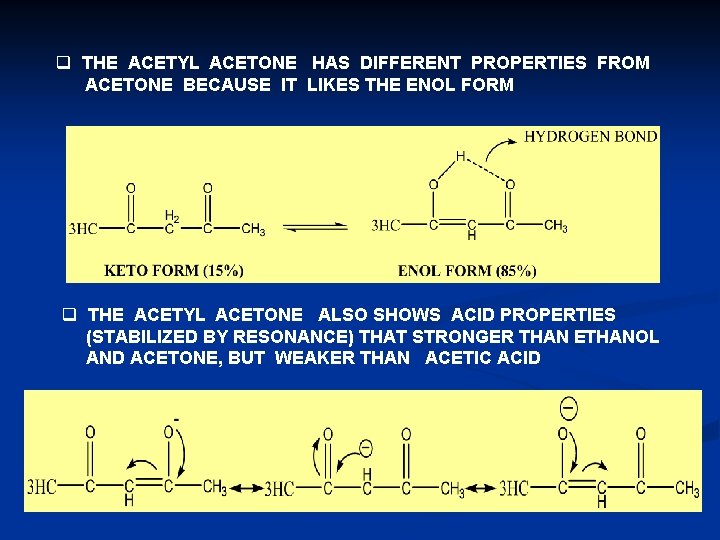
q THE ACETYL ACETONE HAS DIFFERENT PROPERTIES FROM ACETONE BECAUSE IT LIKES THE ENOL FORM q THE ACETYL ACETONE ALSO SHOWS ACID PROPERTIES (STABILIZED BY RESONANCE) THAT STRONGER THAN ETHANOL AND ACETONE, BUT WEAKER THAN ACETIC ACID
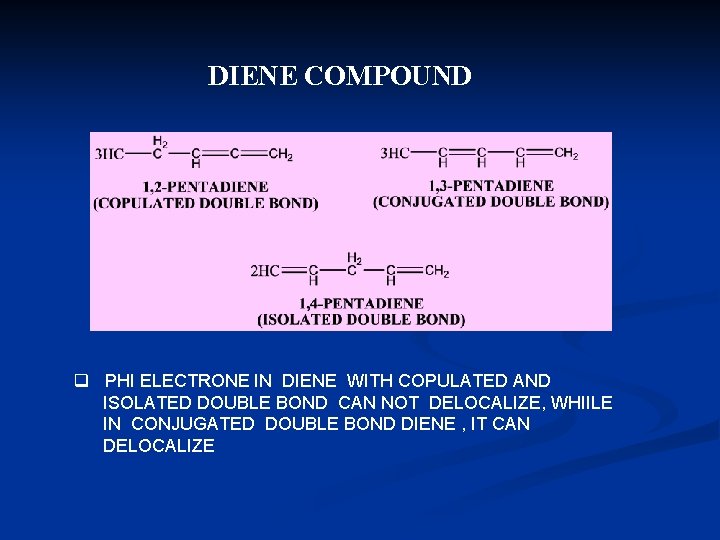
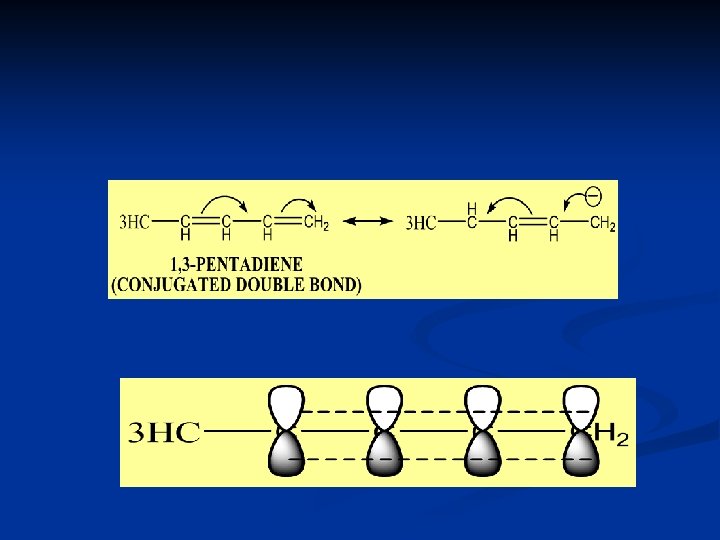
DIENE COMPOUND q PHI ELECTRONE IN DIENE WITH COPULATED AND ISOLATED DOUBLE BOND CAN NOT DELOCALIZE, WHIILE IN CONJUGATED DOUBLE BOND DIENE , IT CAN DELOCALIZE
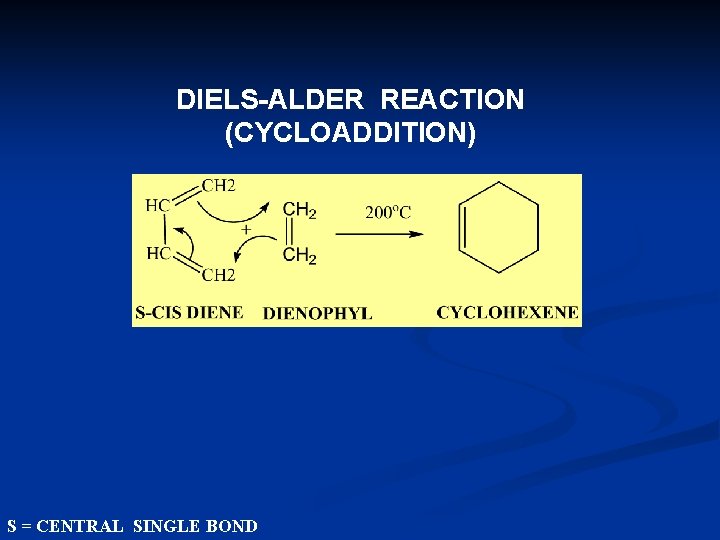
DIELS-ALDER REACTION (CYCLOADDITION) S = CENTRAL SINGLE BOND
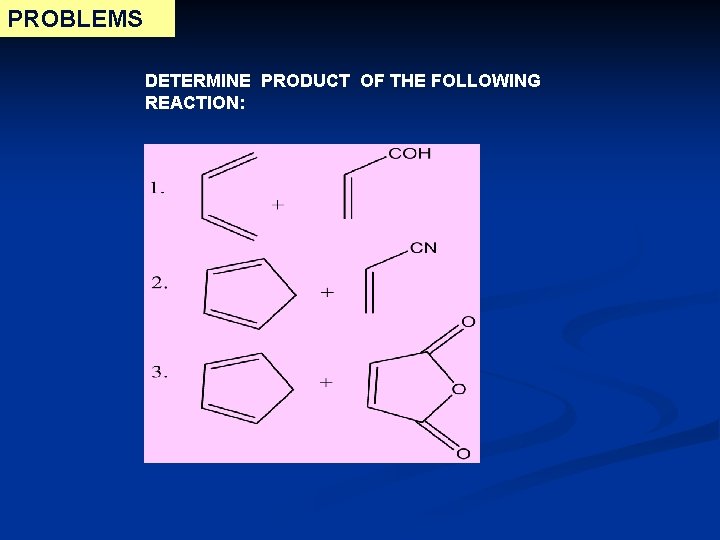
PROBLEMS DETERMINE PRODUCT OF THE FOLLOWING REACTION:
- Slides: 27