POLYESTER History Forms Manufacturing Process Future Definition Polyester









- Slides: 30

POLYESTER ¡ ¡ History Forms Manufacturing Process Future

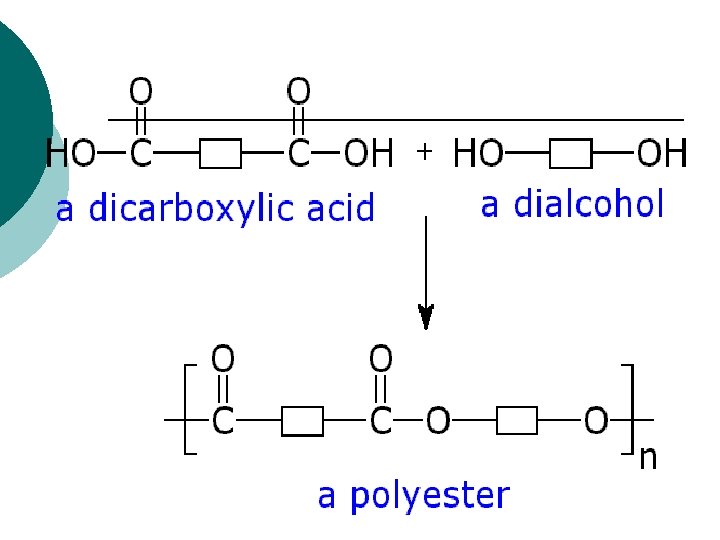
Definition ¡ Polyester (aka Terylene) is a category of polymers which contain the ester functional group in their main chain. Polyester is currently defined as: n“Long chain polymers chemically composed of at least 85% by weight of an ester and a dihydric alcohol and terephthalic acid”. The name “polyester” refers to the linkage of several monomers (esters) within the fiber.

History ¡ ¡ ¡ In 1926, United States-based E. I. du Pont de Nemours and Co. began research into very large molecules and synthetic fibers W. H. Carothers, centered on what became nylon, the first synthetic fiber. 1939 -41, British research chemists took interest in the du Pont studies and conducted their own research in the laboratories of Calico Printers Association, Ltd. This work resulted in the creation of the polyester fiber known in England as Terylene. In 1946, du Pont purchased the right to produce this polyester fiber in the United States. The company conducted some further developmental work, and in 1951, began to market the fiber under the name Dacron

Raw Materials Coal ¡ Air ¡ Water ¡ Petroleum ¡


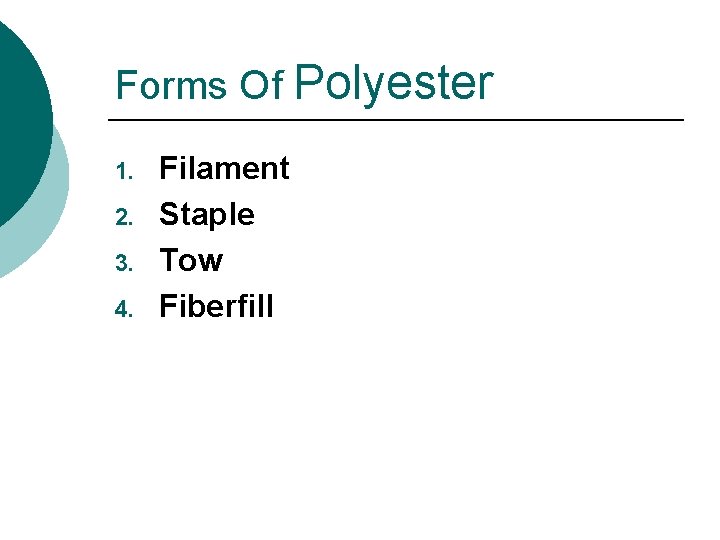
Forms Of Polyester 1. 2. 3. 4. Filament Staple Tow Fiberfill

Uses Of Different Form In Different Places 1. 2. 3. 4. In the filament form, each individual strand of polyester fiber is continuous in length, producing smooth-surfaced fabrics In staple form, filaments are cut to short, predetermined lengths. In this form polyester is easier to blend with other fibers Tow is a form in which continuous filaments are drawn loosely together Fiberfill is the voluminous form used in the manufacture of quilts, pillows, and outerwear


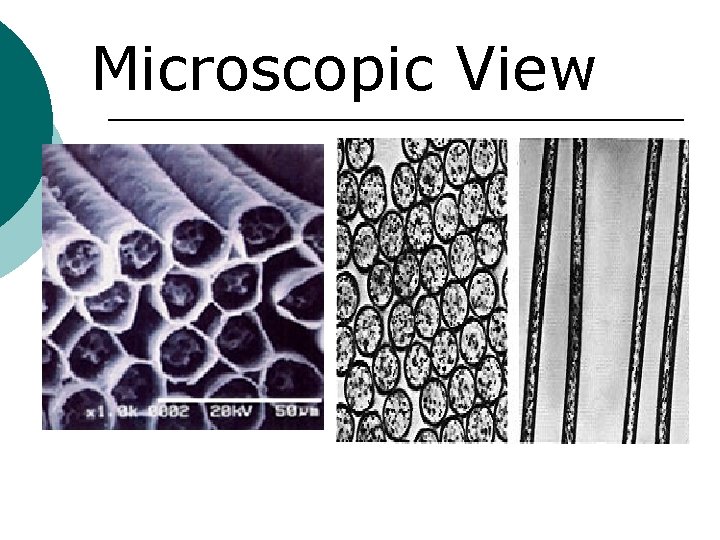
Microscopic View


Different Structures Of Polyester

STRUCTURE AND APPERANCE COLOURLESS AND TRANSPARENT ¡ SMOOTH AND LUSTURUOS ¡ SHAPE AS WE REQIURE ¡ SHINY GLASSROD LIKE ¡

Polyester Fiber Characteristics l l l l l Strong Resistant to stretching and shrinking Resistant to most chemicals Quick drying Crisp and resilient when wet or dry Wrinkle resistant Mildew resistant Abrasion resistant Retains heat-set pleats and crease Easily washed

Polyester Blends ¡ 1. 2. 3. Polyester and Cotton Resist wrinkles Resist stains Retain shape

Polyester Blends ¡ 1. 2. 3. Polyester and Wool Wrinkle resistance Shape retention Increase durability

Polyester Blends ¡ 1. 2. 3. Polyester and Rayon More durable Shape retention More resilience

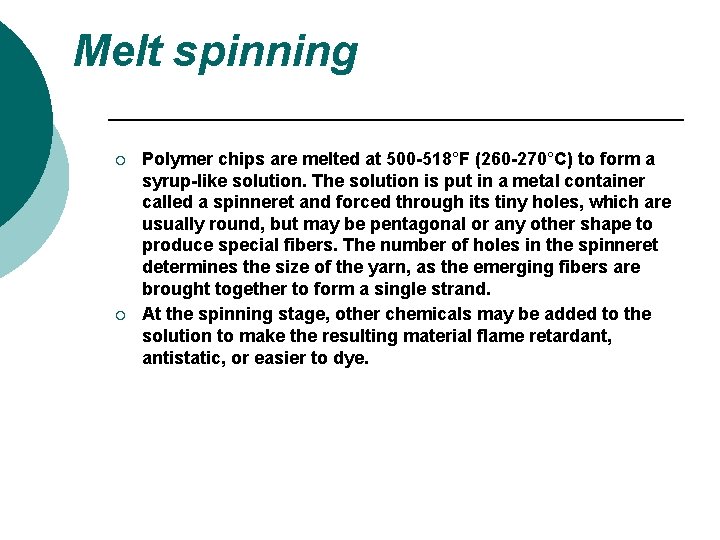
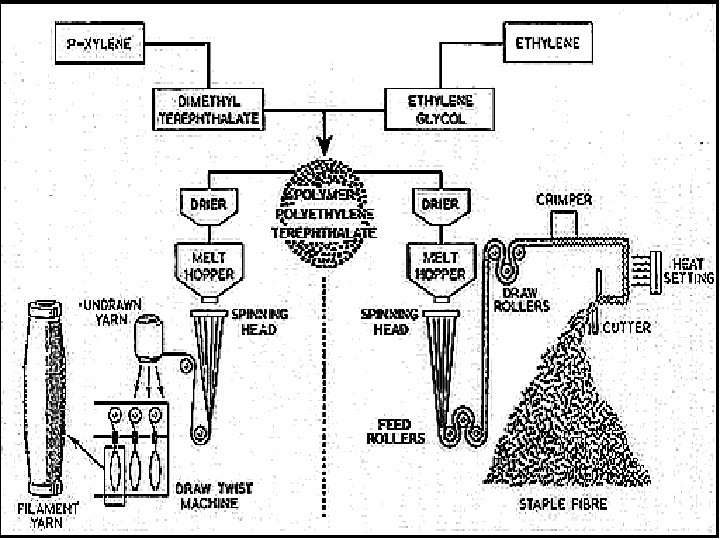
Manufacturing Filament Yarn ¡ ¡ ¡ Polymerization Drying Melt spinning Drawing the fiber Winding

Polymerization ¡ ¡ To form polyester, dimethyl terephthalate is first reacted with ethylene glycol in the presence of a catalyst at a temperature of 302 -410°F (150 -210°C). The resulting chemical, a monomer (single, non-repeating molecule) alcohol, is combined with terephthalic acid and raised to a temperature of 472°F (280°C). Newly-formed polyester, which is clear and molten, is extruded through a slot to form long ribbons. Drying ¡ After the polyester emerges from polymerization, the long molten ribbons are allowed to cool until they become brittle. The material is cut into tiny chips and completely dried to prevent irregularities in consistency.

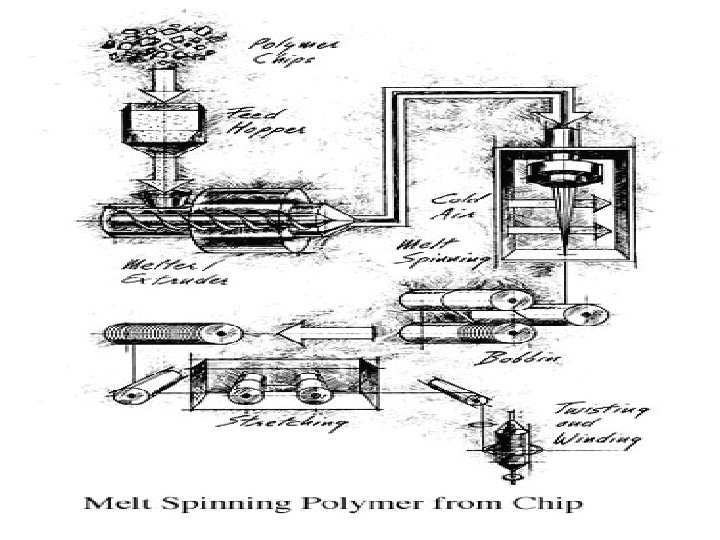
Melt spinning ¡ ¡ Polymer chips are melted at 500 -518°F (260 -270°C) to form a syrup-like solution. The solution is put in a metal container called a spinneret and forced through its tiny holes, which are usually round, but may be pentagonal or any other shape to produce special fibers. The number of holes in the spinneret determines the size of the yarn, as the emerging fibers are brought together to form a single strand. At the spinning stage, other chemicals may be added to the solution to make the resulting material flame retardant, antistatic, or easier to dye.



Drawing the fiber ¡ ¡ When polyester emerges from the spinneret, it is soft and easily elongated up to five times its original length. This increases the strength, tenacity, and resilience of the fiber. This time, when the filaments dry, the fibers become solid and strong instead of brittle. Drawn fibers may vary greatly in diameter and length, Also, as the fibers are drawn, they may be textured or twisted to create softer or duller fabrics. Winding ¡ After the polyester yarn is drawn, it is wound on large bobbins or flat-wound packages, ready to be woven into material.



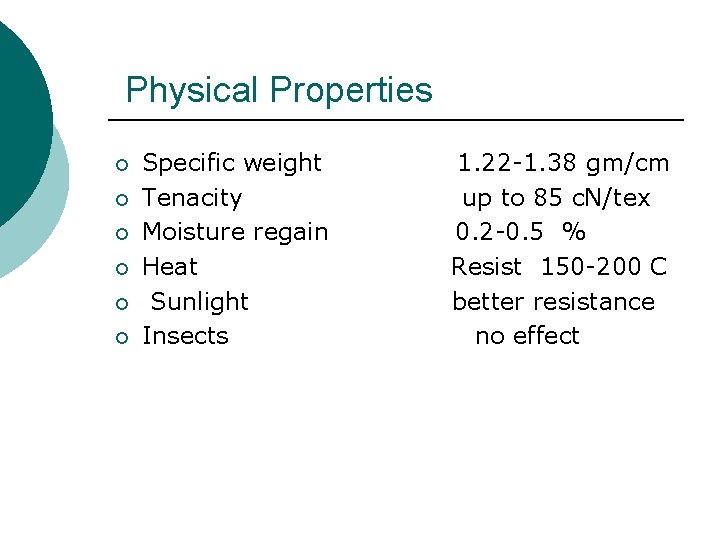
Physical Properties ¡ ¡ ¡ Specific weight Tenacity Moisture regain Heat Sunlight Insects 1. 22 -1. 38 gm/cm up to 85 c. N/tex 0. 2 -0. 5 % Resist 150 -200 C better resistance no effect

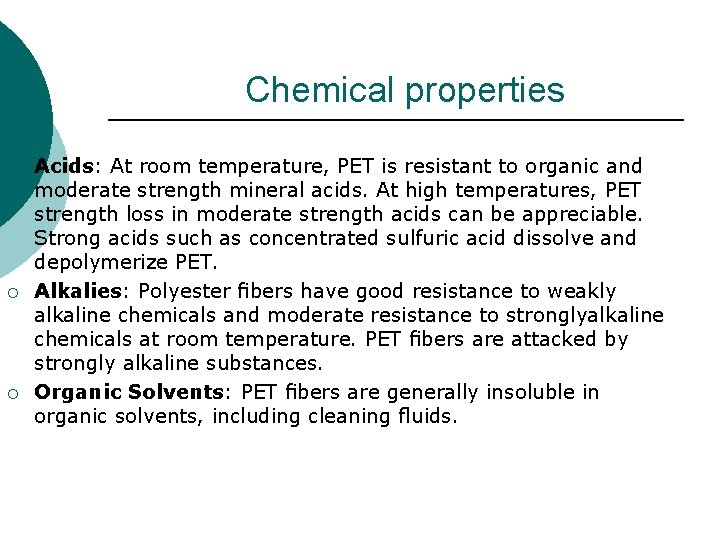
Chemical properties ¡ ¡ ¡ Acids: At room temperature, PET is resistant to organic and moderate strength mineral acids. At high temperatures, PET strength loss in moderate strength acids can be appreciable. Strong acids such as concentrated sulfuric acid dissolve and depolymerize PET. Alkalies: Polyester fibers have good resistance to weakly alkaline chemicals and moderate resistance to stronglyalkaline chemicals at room temperature. PET fibers are attacked by strongly alkaline substances. Organic Solvents: PET fibers are generally insoluble in organic solvents, including cleaning fluids.

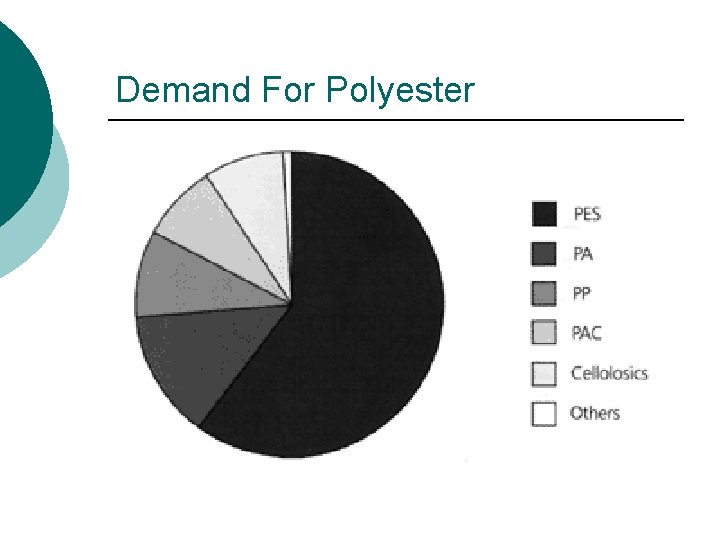
Demand For Polyester

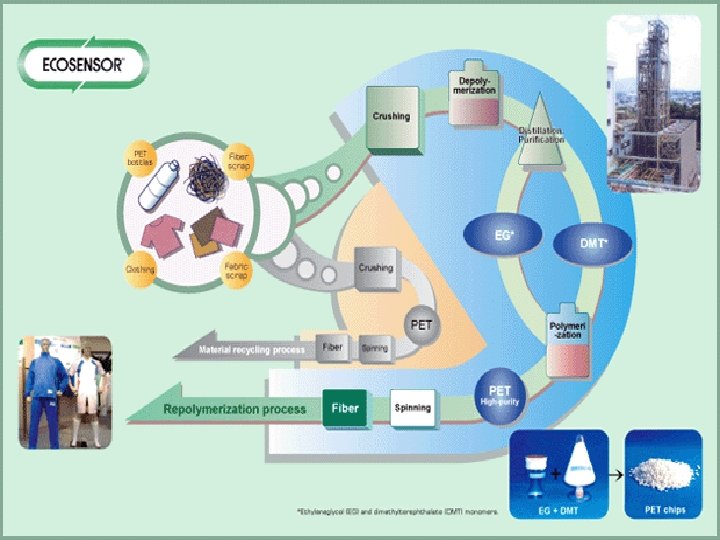
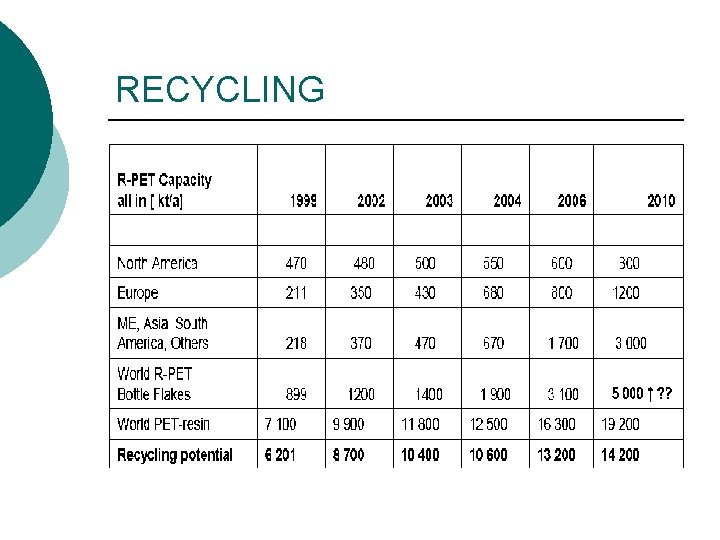
RECYCLING

FUTURE ¡ ¡ Biodegradable and biocompatible poly(3 hydroxybutyrate-co-3 -hydroxyvalerate) (PHBV), a copolymer of microbial polyester, was fabricated as a Nano fibrous mat by electro spinning The researchers have developed a process in which polyester is dramatically strengthened with a material known as a liquid crystalline polymer. The liquid crystalline polymer used in the research is called Vectra , a plastic material similar to Kevlar that is five times stronger than steel. Polyester is used because its chemical structure is ideal for making bonds with the liquid crystalline polymer

Some Major Polyester Fiber Uses ¡ ¡ ¡ Apparel: Every form of clothing Home Furnishings: Carpets, curtains, draperies, sheets and pillow cases, wall coverings, and upholstery Other Uses: hoses, power belting, ropes and nets, thread, tire cord, auto upholstery, sails, floppy disk liners, and fiberfill for various products including pillows and furniture