Polyatomic Nomenclature Polyatomic Nomenclature In polyatomic compounds a


- Slides: 11

Polyatomic Nomenclature

Polyatomic Nomenclature § In polyatomic compounds, a positive ion and negative ion attract one another to form an ionic bond. § To write formulas for polyatomic compound, determine what charge is on the positive ion and what charge is on the negative ion. Then, write the formula so that the compound is neutral… § the ‘criss-cross’ method may be useful

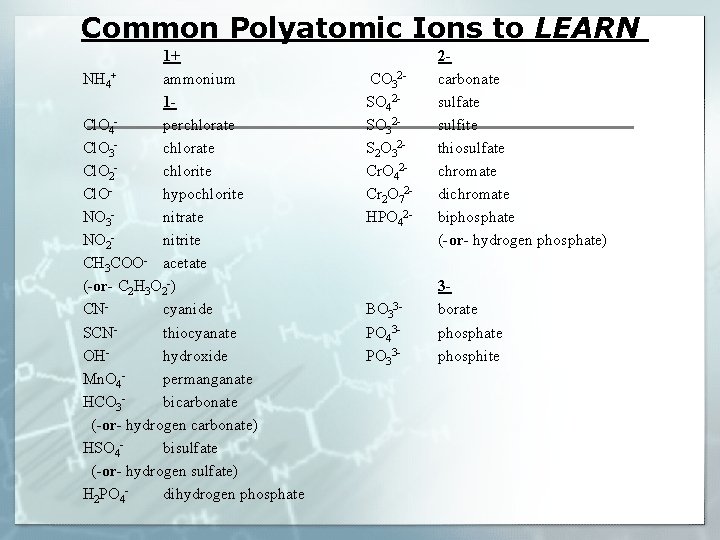
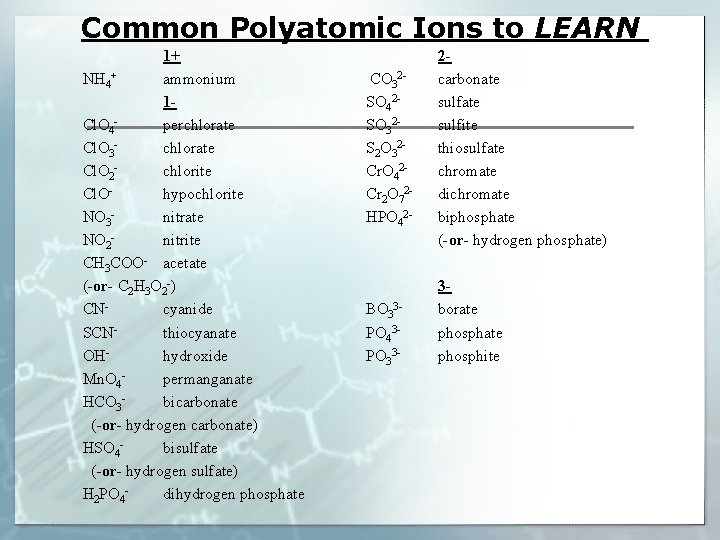
Common Polyatomic Ions to LEARN 1+ NH 4+ ammonium 1 Cl. O 4 perchlorate Cl. O 3 chlorate Cl. O 2 chlorite Cl. Ohypochlorite NO 3 nitrate NO 2 nitrite CH 3 COO- acetate (-or- C 2 H 3 O 2 -) CNcyanide SCNthiocyanate OHhydroxide Mn. O 4 permanganate HCO 3 bicarbonate (-or- hydrogen carbonate) HSO 4 bisulfate (-or- hydrogen sulfate) H 2 PO 4 dihydrogen phosphate CO 32 SO 42 SO 32 S 2 O 32 Cr. O 42 Cr 2 O 72 HPO 42 - 2 carbonate sulfite thiosulfate chromate dichromate biphosphate (-or- hydrogen phosphate) BO 33 PO 43 PO 33 - 3 borate phosphite

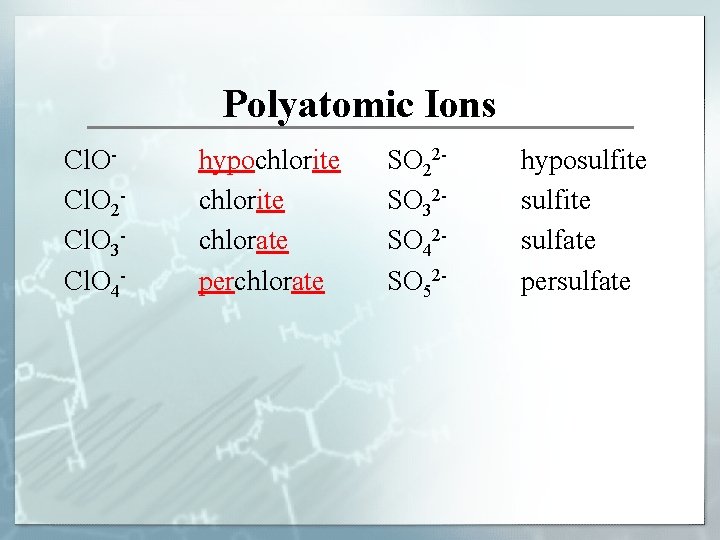
Polyatomic Ions Cl. O 2 Cl. O 3 Cl. O 4 - hypochlorite chlorate perchlorate SO 22 SO 32 SO 42 SO 52 - hyposulfite sulfate persulfate

Polyatomic Nomenclature § For example… magnesium nitrate § forms from: § the magnesium ion, Mg 2+ § and the nitrate ion, NO 3 - § So, the formula is Mg(NO 3)2

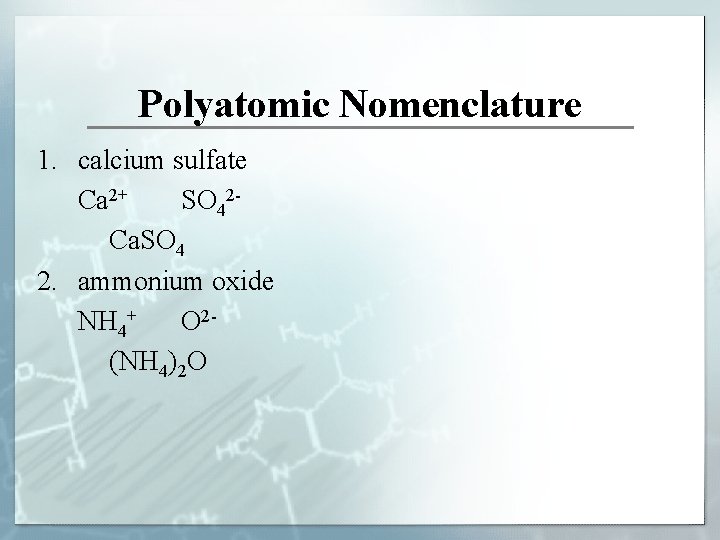
Polyatomic Nomenclature 1. calcium sulfate Ca 2+ SO 42 Ca. SO 4 2. ammonium oxide NH 4+ O 2(NH 4)2 O

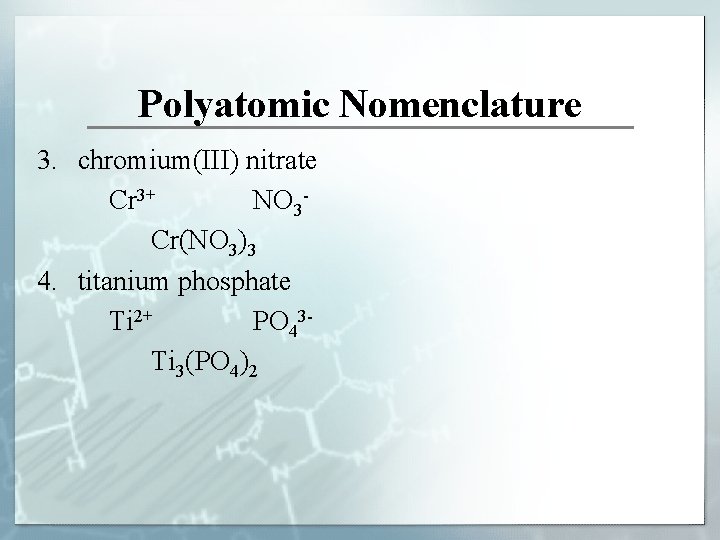
Polyatomic Nomenclature 3. chromium(III) nitrate Cr 3+ NO 3 Cr(NO 3)3 4. titanium phosphate Ti 2+ PO 43 Ti 3(PO 4)2

Ionic Nomenclature § Ionic bonding occurs between… § a metal & a non-metal § To name an ionic compound: § name the metal (name the cation) § name the non-metal, adding an –ide ending (name the anion)

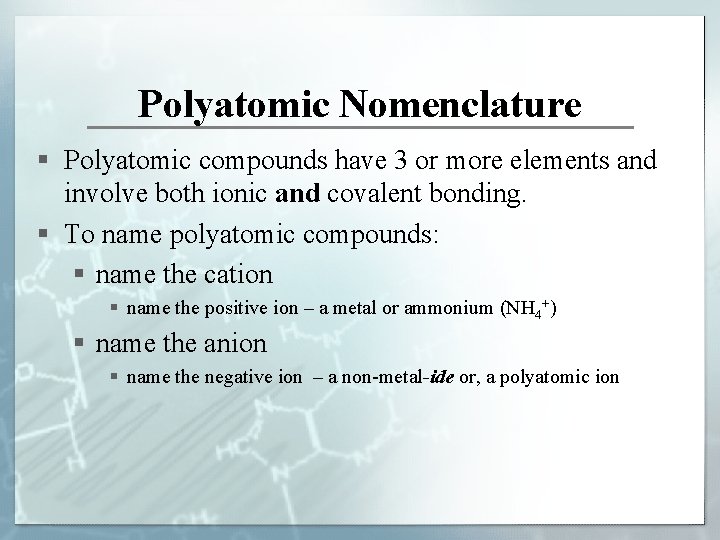
Polyatomic Nomenclature § Polyatomic compounds have 3 or more elements and involve both ionic and covalent bonding. § To name polyatomic compounds: § name the cation § name the positive ion – a metal or ammonium (NH 4+) § name the anion § name the negative ion – a non-metal-ide or, a polyatomic ion

Common Polyatomic Ions to LEARN 1+ NH 4+ ammonium 1 Cl. O 4 perchlorate Cl. O 3 chlorate Cl. O 2 chlorite Cl. Ohypochlorite NO 3 nitrate NO 2 nitrite CH 3 COO- acetate (-or- C 2 H 3 O 2 -) CNcyanide SCNthiocyanate OHhydroxide Mn. O 4 permanganate HCO 3 bicarbonate (-or- hydrogen carbonate) HSO 4 bisulfate (-or- hydrogen sulfate) H 2 PO 4 dihydrogen phosphate CO 32 SO 42 SO 32 S 2 O 32 Cr. O 42 Cr 2 O 72 HPO 42 - 2 carbonate sulfite thiosulfate chromate dichromate biphosphate (-or- hydrogen phosphate) BO 33 PO 43 PO 33 - 3 borate phosphite

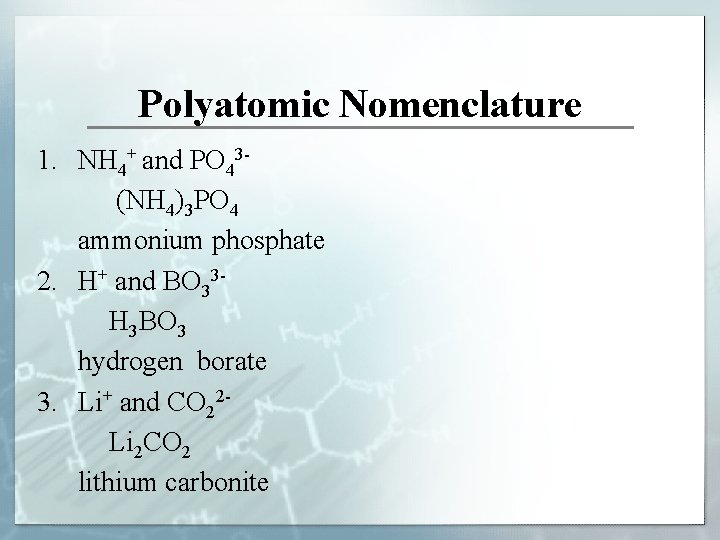
Polyatomic Nomenclature 1. NH 4+ and PO 43 (NH 4)3 PO 4 ammonium phosphate 2. H+ and BO 33 H 3 BO 3 hydrogen borate 3. Li+ and CO 22 Li 2 CO 2 lithium carbonite