Pollution Pollution defined as n n n Something




























- Slides: 37

Pollution

Pollution defined as n n n Something that is unwanted that interferes with the environment and an organisms self sustaining processes in a negative way. The texts A change in the physical chemical and biological characteristics of air water and food that adversely effects the survival of organisms.

Basic Toxicology

Basic Toxicology Toxin=Damages a living organism by ingesting or absorbed. n Toxicity=the degree which a toxin harmful. n Any thing in high enough quantities may be harmful n l Ex water

Factors to consider Dosage n Amount of exposure n Size and age or organism n Ability of organism to detoxify n Sensitivity to substance n Synergistic/Antagistic effects n

Dose response analysis n 2 tests l LD 50 when 50% die n l Poisons-LD 50= 50 mg or less Threshold dose=when a negative effect occurs

Types of effects n Acute-Short exposure to a high level of toxin l n Ex snake bite Chronic-Long term exposure to a low level toxin.

Risk assessment/management Assessment calculations of the risk taken n Management=strategies taken to reduce risk n

Air Pollution

Air Pollution n 2 sources human and natural Natural=volcano l Car=man The majority of the pollution today is from man and occurred after the industrial revolution. l

Beijing is one of the most polluted cities

Sources n Primary pollutants released directly into the lower atmosphere and are toxic. l n Ex mercury coal burning Secondary-toxins formed in the atmosphere from multiple primary pollutants. l SO 2 from coal +H 2 O H 2 SO 4

EPA n n n Environmental Protection Agency Regulates pollution Regulates 6 “Criteria Pollutants” l l l Carbon monoxide CO-combustion-60% car Lead Pb-from coal and smelting Ozone O 3 -good in stratosphere-bad in troposphere-from combustion. Nitrogen Dioxide NO 2 -car engines forms nitric acid Sulfur dioxide SO 2 coal and diesel–sulfuric acid Particulates

Changes Since 1970 CO and lead have decreased n New concerns=VOC volatile organic compounds. n l Toxic organic compounds from manufacturing, dry cleaning, industrial solvents, form O 3

Smog n Fog=natural water vaper l n 2 types industrial and photochemical Smog=Toxic gas

Industrial Smog

n Industrial (grey) smog- formed from the burning of coal common 19001950. CO 2 combines with particulate matter. l Problems=Sulfuric acid lead particulate matter. l Lead to- pneumonia, tuberculosis, heart failure, bronchitis, whooping cough. 1952 killed 4000 in London l

Photochemical smog

n Photochemical Smog. The brown cloud found on sunny days. l Caused by car combustion and industrial combustion. l VOC, NOx, O 3 combine, catalyzed by sunlight, to form a toxic gas. l n Common in baltimore

Inversion n Air is trapped by warmer layers of air in low places.

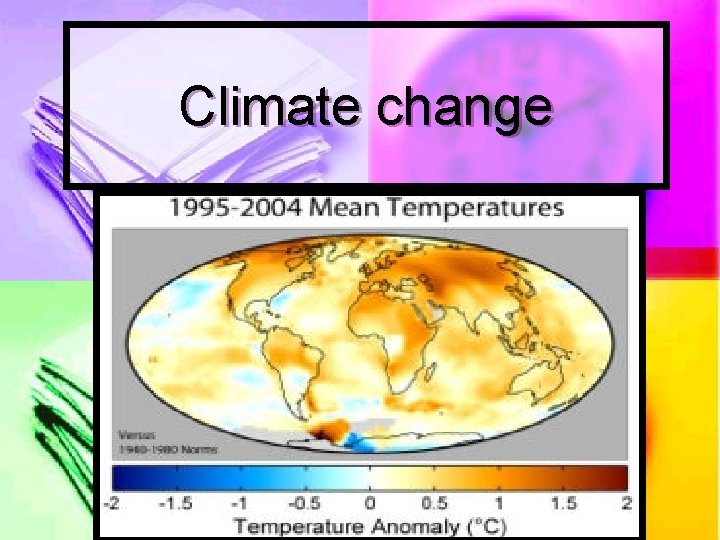
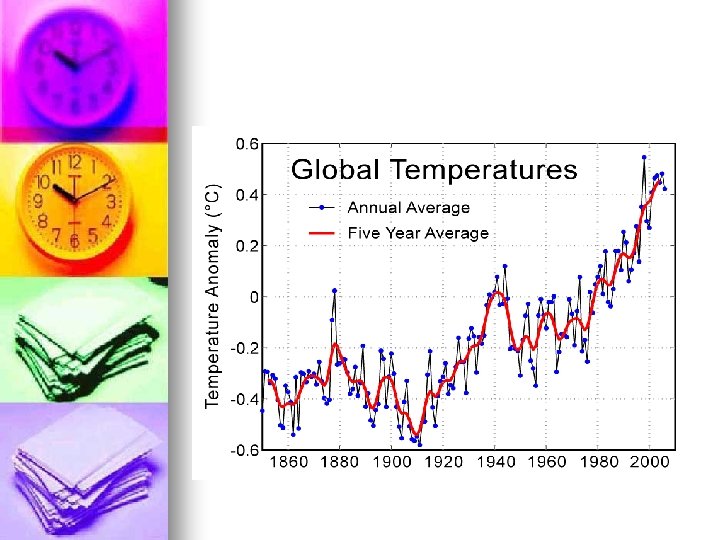
Climate change

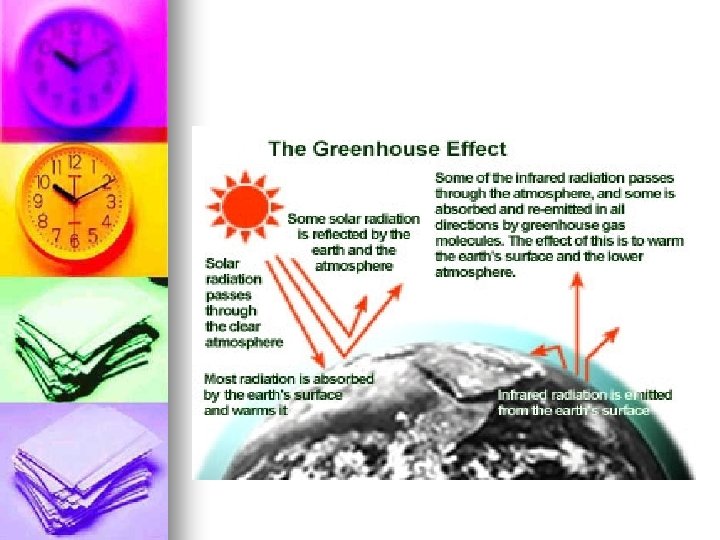
Caused By Increasing CO 2 and other green house gasses cause the green house effect. n 1900 -1950 25% caused by combustion 75% by reducing biomass. n 1950 -today 75% by combustion 25% by reduction in biomass. n

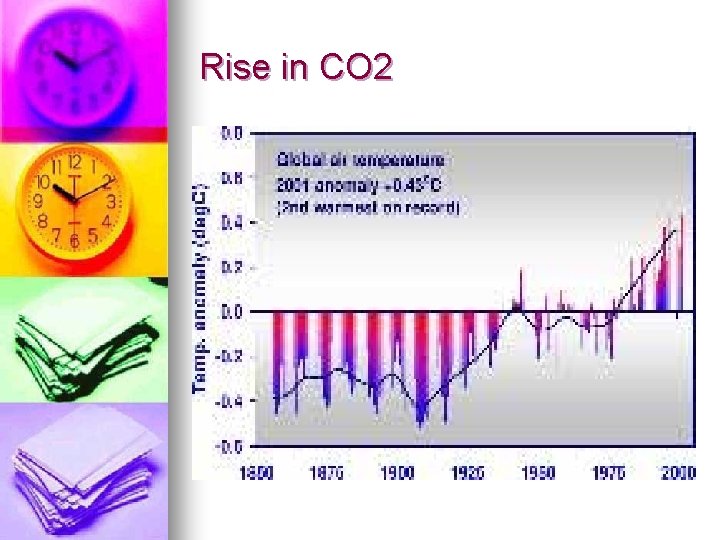
IPCC (Intergovernmental panel on climate change) report 1 -Climate change is occurring n There is a correlation between temperature and CO 2 levels (greater than 90%) n CO 2 1950 280 ppm 2006 380 ppm n Methane 1950 715 ppm 2005 1774 ppm n NOx 270 -319 n


Rise in CO 2


Effects Additive effect decrease in ice caps= increased temperatures. n Increased heat = increased H 2 O gas=increased heat. n Increased temp=less permafrost=more Co 2 released from the permafrost. n Increased temp= decreased land= less biota and more CO 2 n

Impact Higher levels of water-maryland 4 th most at risk n Decreasing glaciers=less drinking water n Increased storms n

Ozone depletion

Ozone depletion n Ozone –in stratosphere is a toxin but in the stratosphere provides a defense from UV radiation. l Formation n 3 O 2 + UV sunlight 2 O 3

Ozone depletion Occurs during the summer months. n Caused by Chloroflurocarbons (CFCs) And other compounds used in propellants, fire extinguishers, and hair spray. n

Reaction CFCs break down to Chlorine gas and reacts with ozone n Cl + O 3 O 2+Cl. O n is stored in ice crystals over the winter. n In the spring the crystals melt and continue to react n Cl. O+O Cl+O 2 n

Results 26 million square Km zone of thinness (hole). n Chronic Exposure= eye cataracts, skin cancer, weakening of immune system. n Kill phytoplankton, -primary producers. n

Acid rain

Acid rain: Formed from SO 2 and NOx and water into sulfuric and nitric acids n p. H=range from 5. 4 -2. 3 n Caused by coal and auto emmissions n

Environmental effects n n n n Mineral leaching Sulfur and nitrogen buildup in soil/lakes Increased aluminum buildup Leaching of calcium from conifers Lowering of p. H in lakes Human respiratory irritation Dissolving stone-monuments buildings exc.

2 types Dry acidic particle depositiondrops few days after n Wet deposition-drops 4 -14 days n 1990 Clean air Act reduced SO 2 and NOx levels. n