Pollutants and environmental compartments 1ii Physicochemical properties of

- Slides: 48

Pollutants and environmental compartments 1(ii) Physico-chemical properties of pollutants and their influence on their behaviour in the environment

Aims • To provide overview of molecular properties of pollutants in the environment: – Vapour pressure – theoretical background, molecular interactions governing vapour pressure, availability of experimental vapour pressure data and estimation methods – Activity coefficient and solubility in water – thermodynamic consideration, effect of temperature and solution composition on aqueous solubility and activity coefficients, availability of experimental data and estimation methods Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 2

Outcomes • Students will be able to: – estimate relevant physico-chemical properties of pollutants from their structure – predict reactivity of pollutants and possible environmental behavior of pollutants Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 3

Vapour pressure • Definition: – Pressure of a substance in equilibrium with its pure condensed (liquid or solid) phase – pº • Why is it important? – Air/water partitioning – Air/solid partitioning • When is it important? – Spills – Pesticide application Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 4

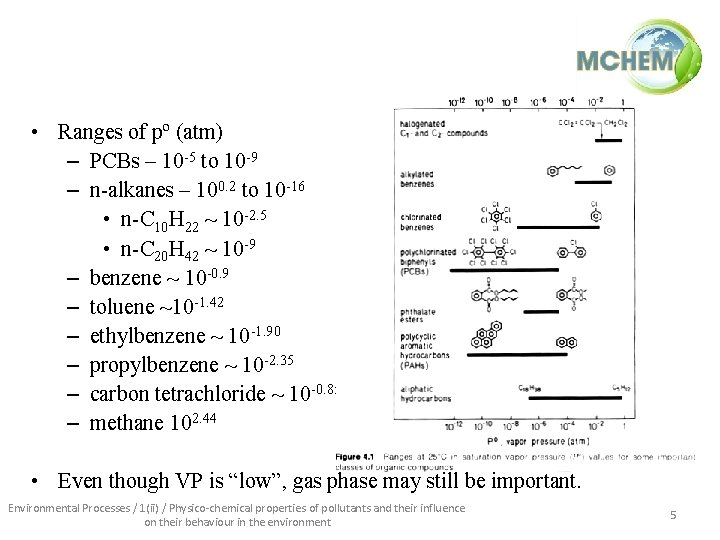
• Ranges of pº (atm) – PCBs – 10 -5 to 10 -9 – n-alkanes – 100. 2 to 10 -16 • n-C 10 H 22 ~ 10 -2. 5 • n-C 20 H 42 ~ 10 -9 – benzene ~ 10 -0. 9 – toluene ~10 -1. 42 – ethylbenzene ~ 10 -1. 90 – propylbenzene ~ 10 -2. 35 – carbon tetrachloride ~ 10 -0. 85 – methane 102. 44 • Even though VP is “low”, gas phase may still be important. Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 5

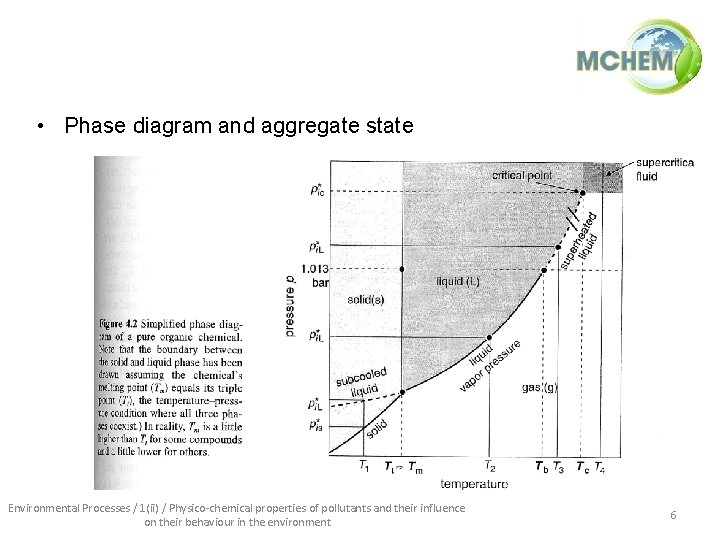
• Phase diagram and aggregate state Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 6

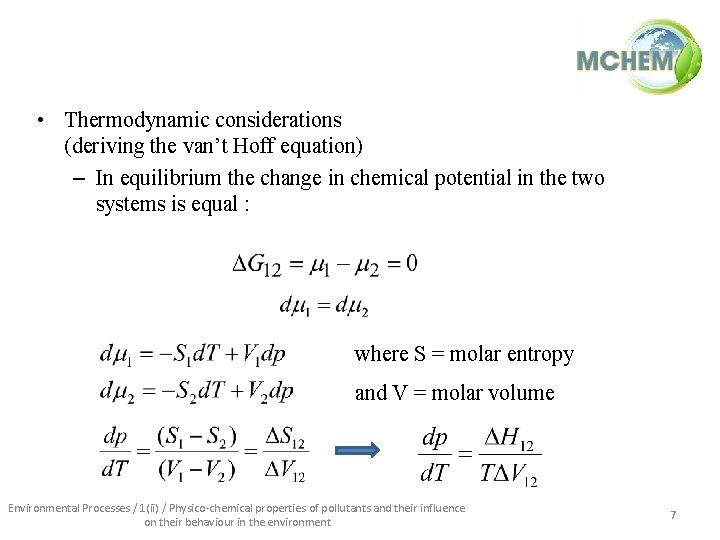
• Thermodynamic considerations (deriving the van’t Hoff equation) – In equilibrium the change in chemical potential in the two systems is equal : where S = molar entropy and V = molar volume Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 7

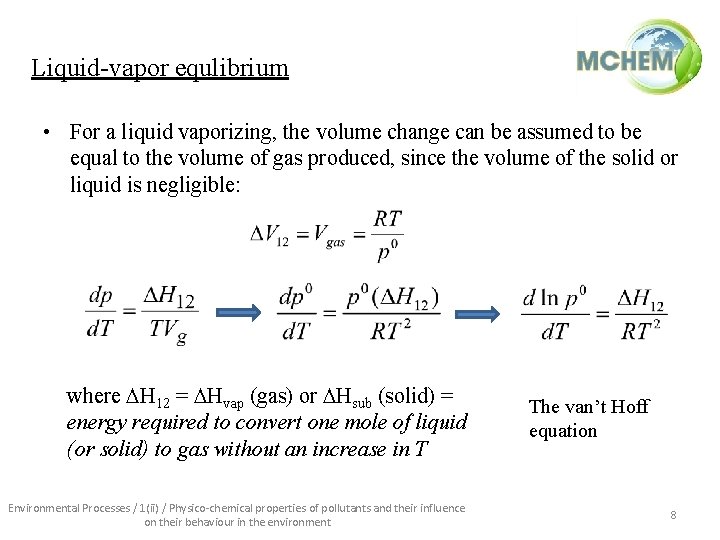
Liquid-vapor equlibrium • For a liquid vaporizing, the volume change can be assumed to be equal to the volume of gas produced, since the volume of the solid or liquid is negligible: where H 12 = Hvap (gas) or Hsub (solid) = energy required to convert one mole of liquid (or solid) to gas without an increase in T Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment The van’t Hoff equation 8

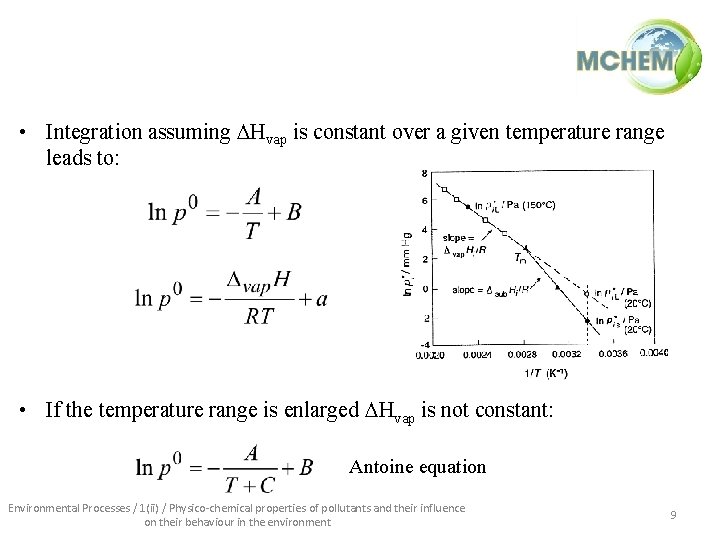
• Integration assuming Hvap is constant over a given temperature range leads to: • If the temperature range is enlarged Hvap is not constant: Antoine equation Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 9

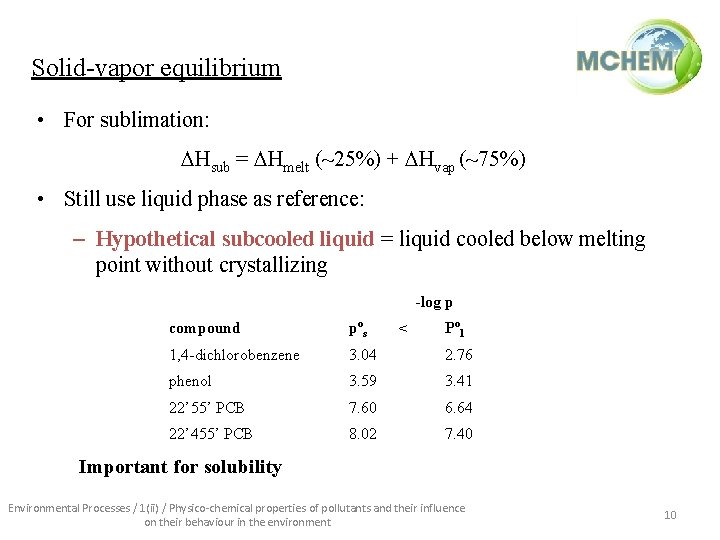
Solid-vapor equilibrium • For sublimation: Hsub = Hmelt (~25%) + Hvap (~75%) • Still use liquid phase as reference: – Hypothetical subcooled liquid = liquid cooled below melting point without crystallizing -log p compound pºs < Pºl 1, 4 -dichlorobenzene 3. 04 2. 76 phenol 3. 59 3. 41 22’ 55’ PCB 7. 60 6. 64 22’ 455’ PCB 8. 02 7. 40 Important for solubility Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 10

Molecular interactions affecting vapor pressure • Molecule: molecule interactions in condensed phase (l or s) have greatest affect on VP: – strong interactions lead to large Hvap, low VP – weak interactions lead to small Hvap, high VP • Intermolecular interactions can be classified into three types: – van der Waals forces (nonpolar) – Polar forces – Hydrogen bonding Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 11

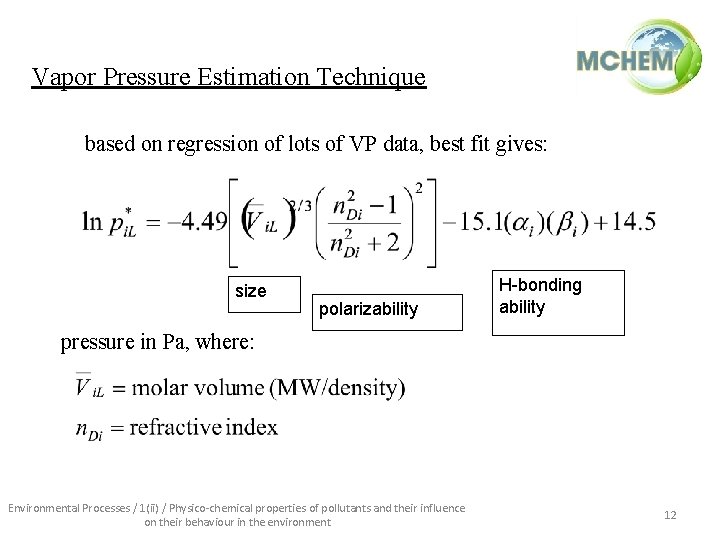
Vapor Pressure Estimation Technique based on regression of lots of VP data, best fit gives: size polarizability H-bonding ability pressure in Pa, where: Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 12

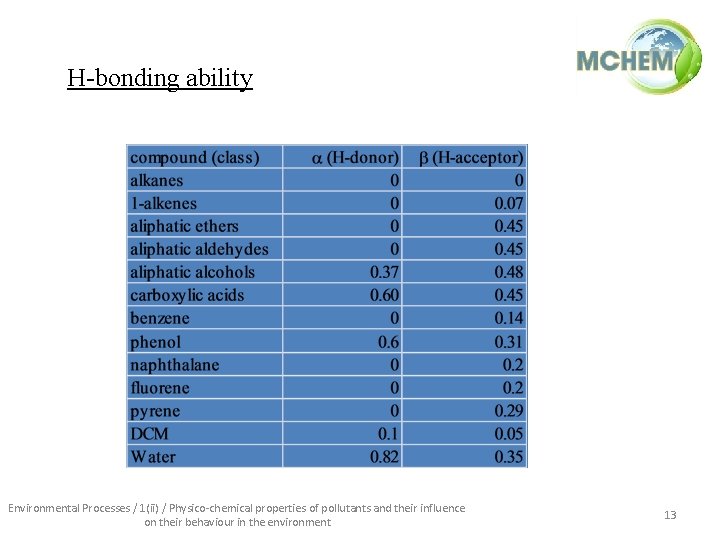
H-bonding ability Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 13

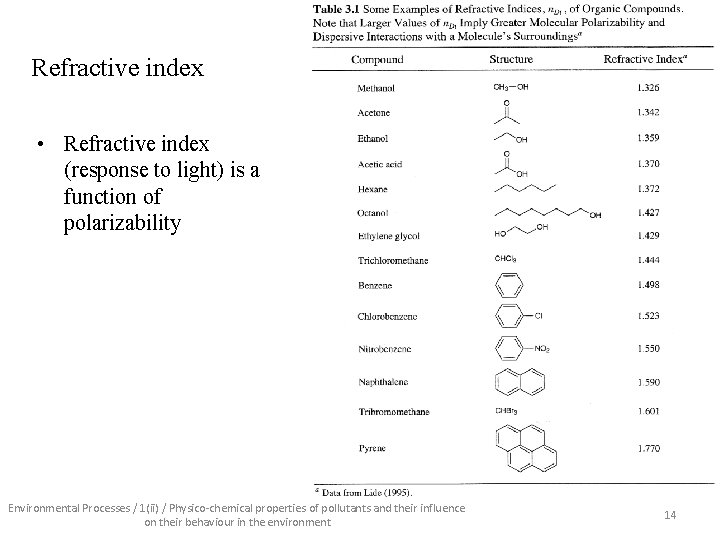
Refractive index • Refractive index (response to light) is a function of polarizability Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 14

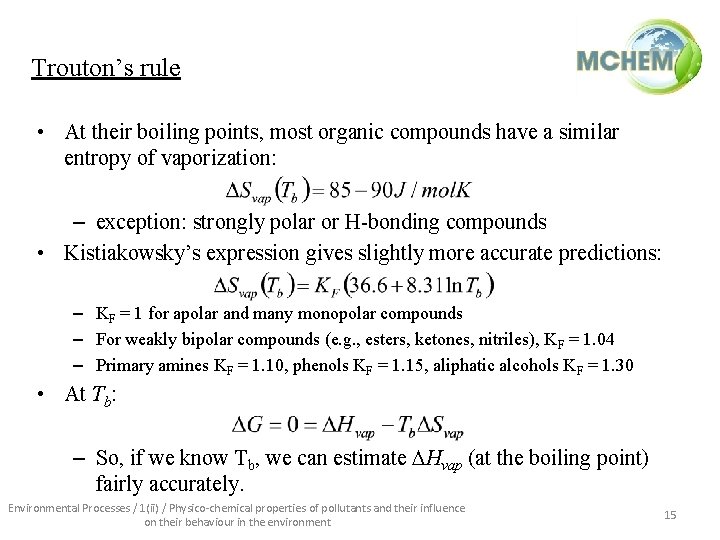
Trouton’s rule • At their boiling points, most organic compounds have a similar entropy of vaporization: – exception: strongly polar or H-bonding compounds • Kistiakowsky’s expression gives slightly more accurate predictions: – KF = 1 for apolar and many monopolar compounds – For weakly bipolar compounds (e. g. , esters, ketones, nitriles), KF = 1. 04 – Primary amines KF = 1. 10, phenols KF = 1. 15, aliphatic alcohols KF = 1. 30 • At Tb: – So, if we know Tb, we can estimate Hvap (at the boiling point) fairly accurately. Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 15

Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 16

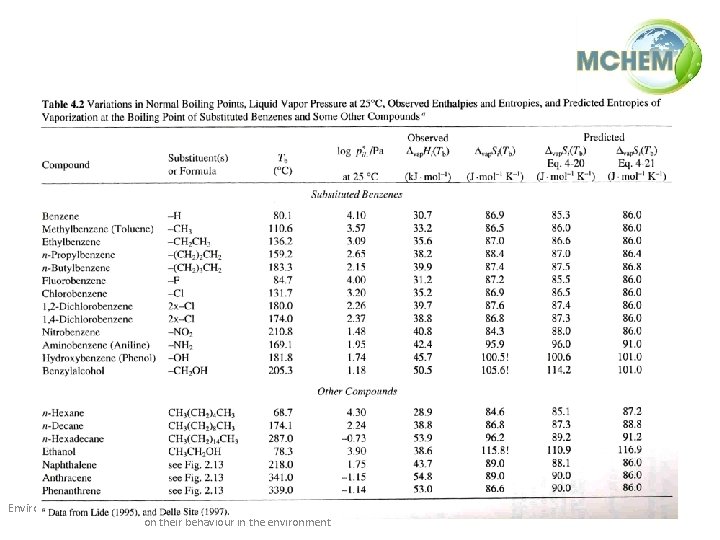
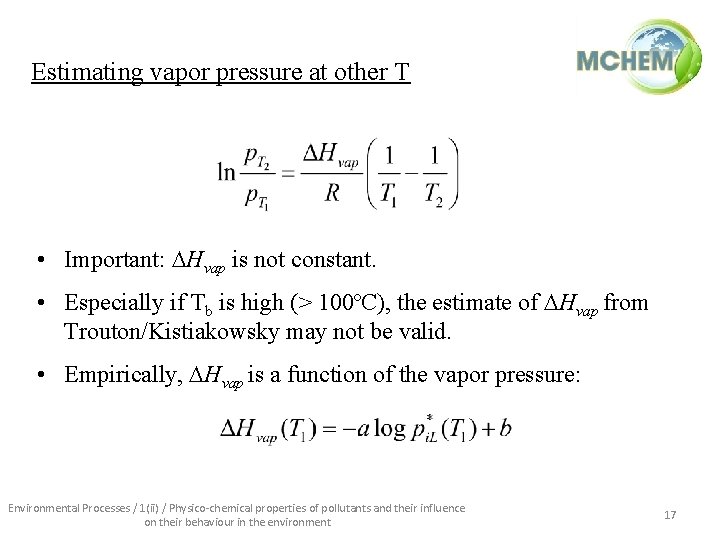
Estimating vapor pressure at other T • Important: Hvap is not constant. • Especially if Tb is high (> 100ºC), the estimate of Hvap from Trouton/Kistiakowsky may not be valid. • Empirically, Hvap is a function of the vapor pressure: Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 17

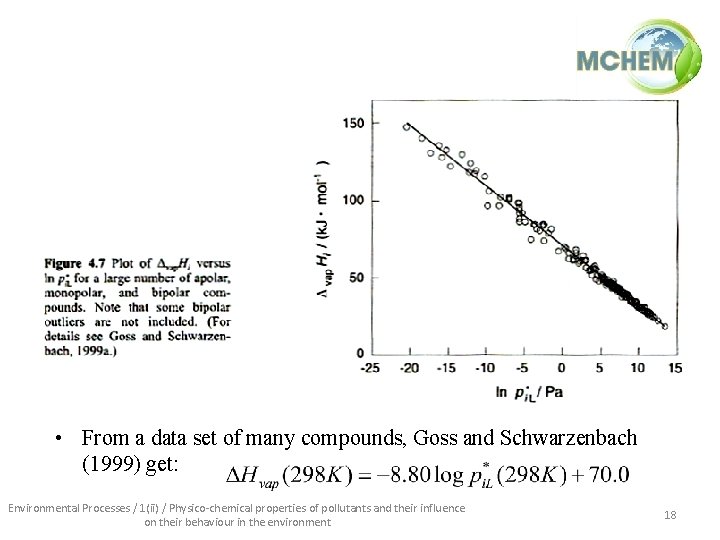
• From a data set of many compounds, Goss and Schwarzenbach (1999) get: Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 18

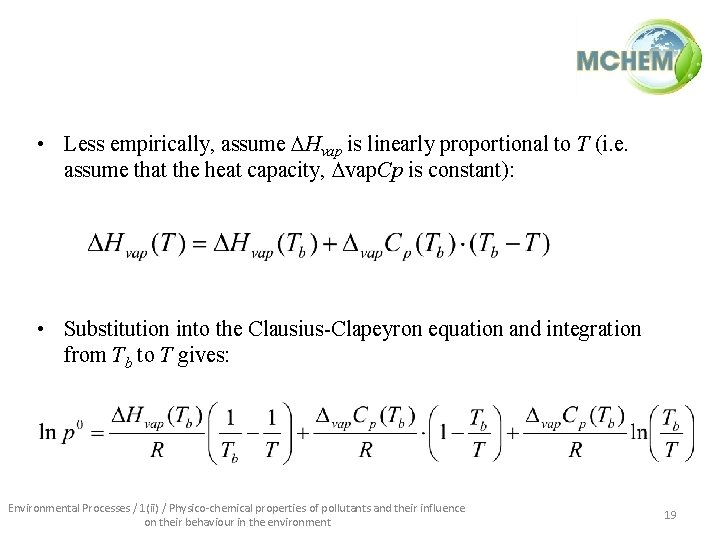
• Less empirically, assume Hvap is linearly proportional to T (i. e. assume that the heat capacity, vap. Cp is constant): • Substitution into the Clausius-Clapeyron equation and integration from Tb to T gives: Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 19

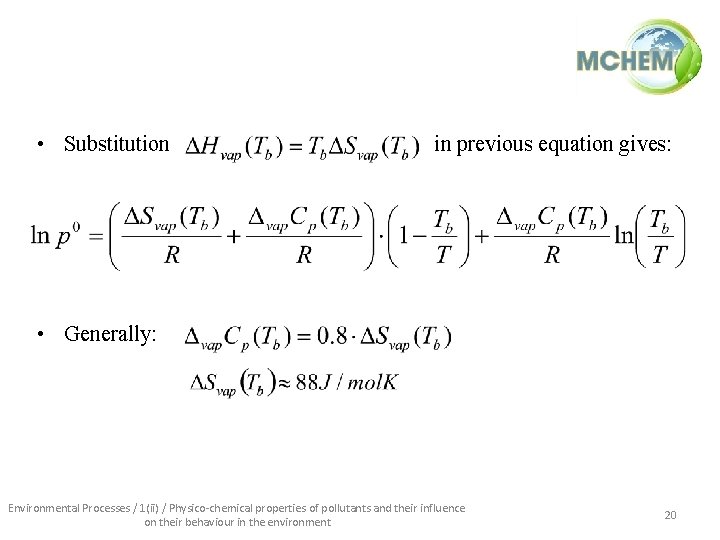
• Substitution in previous equation gives: • Generally: Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 20

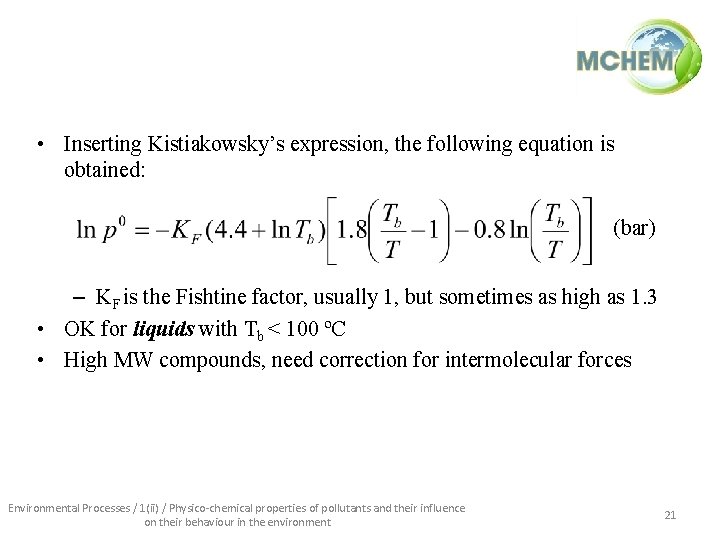
• Inserting Kistiakowsky’s expression, the following equation is obtained: (bar) – KF is the Fishtine factor, usually 1, but sometimes as high as 1. 3 • OK for liquids with Tb < 100 ºC • High MW compounds, need correction for intermolecular forces Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 21

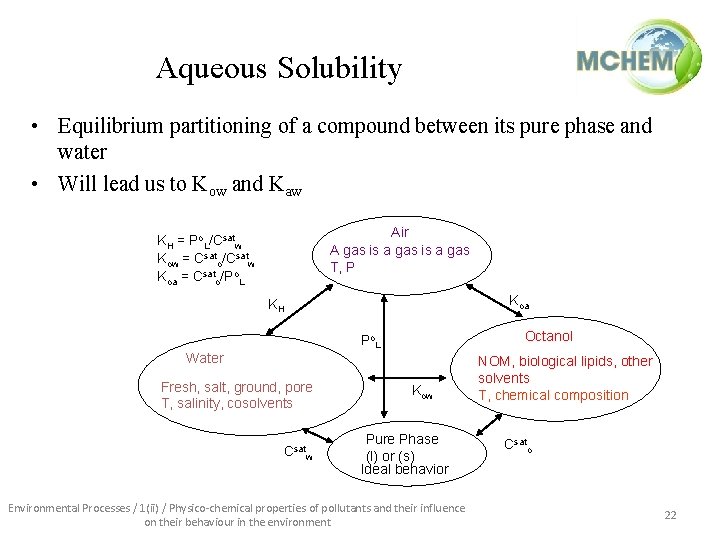
Aqueous Solubility • Equilibrium partitioning of a compound between its pure phase and water • Will lead us to Kow and Kaw Air A gas is a gas T, P KH = Po. L/Csatw Kow = Csato/Csatw Koa = Csato/Po. L Koa KH Octanol Po. L Water Fresh, salt, ground, pore T, salinity, cosolvents Csatw Kow Pure Phase (l) or (s) Ideal behavior Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment NOM, biological lipids, other solvents T, chemical composition Csato 22

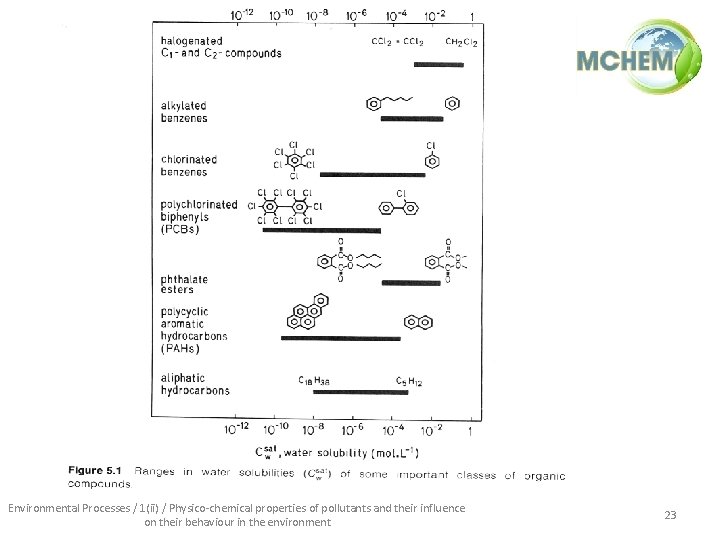
Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 23

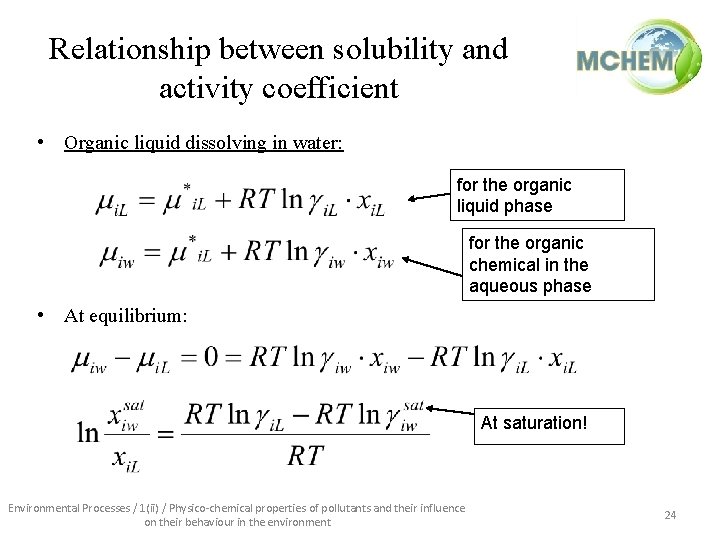
Relationship between solubility and activity coefficient • Organic liquid dissolving in water: for the organic liquid phase for the organic chemical in the aqueous phase • At equilibrium: At saturation! Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 24

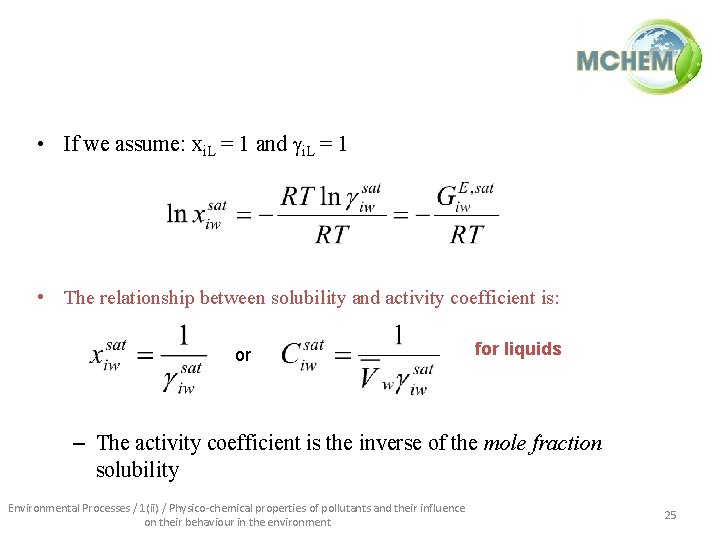
• If we assume: xi. L = 1 and gi. L = 1 • The relationship between solubility and activity coefficient is: or for liquids – The activity coefficient is the inverse of the mole fraction solubility Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 25

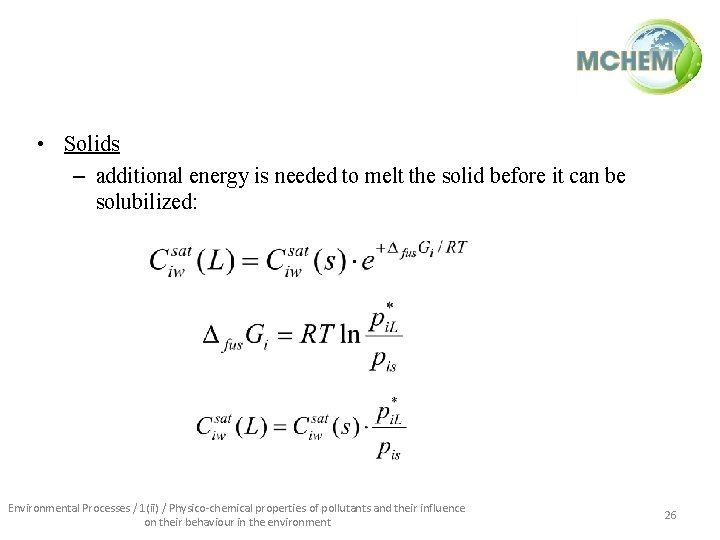
• Solids – additional energy is needed to melt the solid before it can be solubilized: Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 26

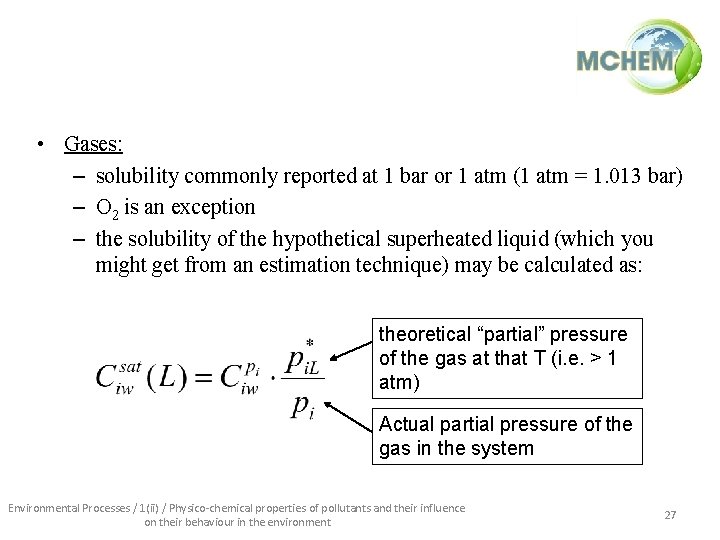
• Gases: – solubility commonly reported at 1 bar or 1 atm (1 atm = 1. 013 bar) – O 2 is an exception – the solubility of the hypothetical superheated liquid (which you might get from an estimation technique) may be calculated as: theoretical “partial” pressure of the gas at that T (i. e. > 1 atm) Actual partial pressure of the gas in the system Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 27

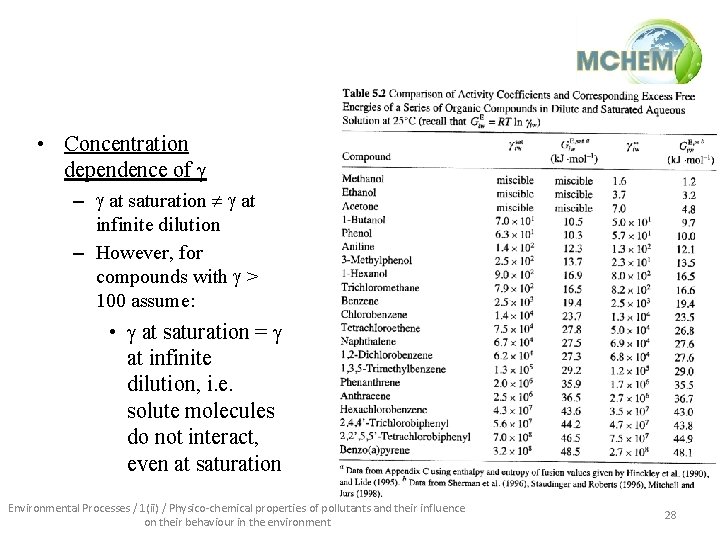
• Concentration dependence of g – g at saturation g at infinite dilution – However, for compounds with g > 100 assume: • g at saturation = g at infinite dilution, i. e. solute molecules do not interact, even at saturation Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 28

Molecular picture of the dissolution process • The two most important driving forces in determining the extent of dissolution of a substance in any liquid solvent are: – an increase in entropy of the system – compatibility of intermolecular forces. Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 29

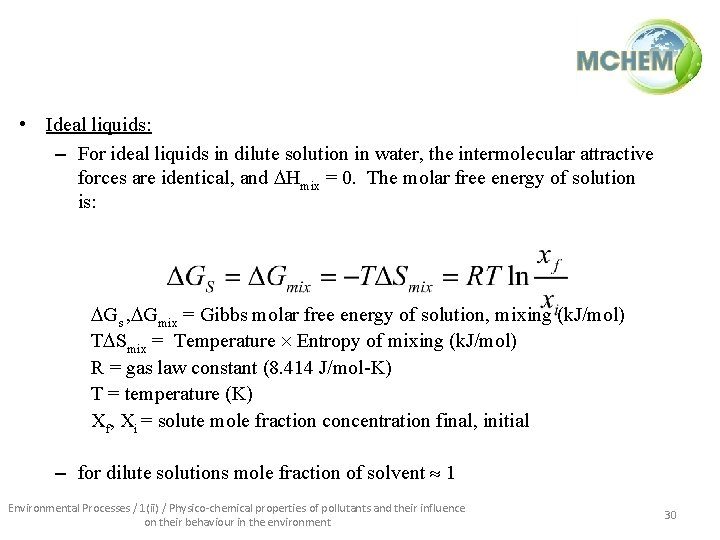
• Ideal liquids: – For ideal liquids in dilute solution in water, the intermolecular attractive forces are identical, and Hmix = 0. The molar free energy of solution is: Gs , Gmix = Gibbs molar free energy of solution, mixing (k. J/mol) T Smix = Temperature Entropy of mixing (k. J/mol) R = gas law constant (8. 414 J/mol-K) T = temperature (K) Xf, Xi = solute mole fraction concentration final, initial – for dilute solutions mole fraction of solvent 1 Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 30

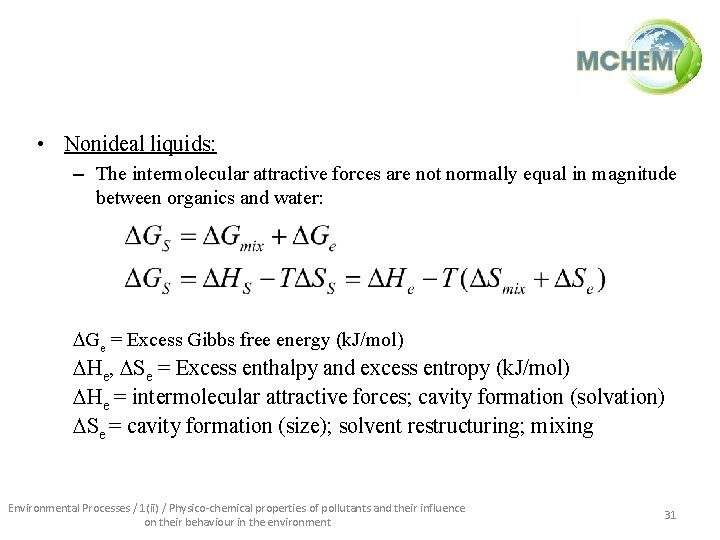
• Nonideal liquids: – The intermolecular attractive forces are not normally equal in magnitude between organics and water: Ge = Excess Gibbs free energy (k. J/mol) He, Se = Excess enthalpy and excess entropy (k. J/mol) He = intermolecular attractive forces; cavity formation (solvation) Se = cavity formation (size); solvent restructuring; mixing Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 31

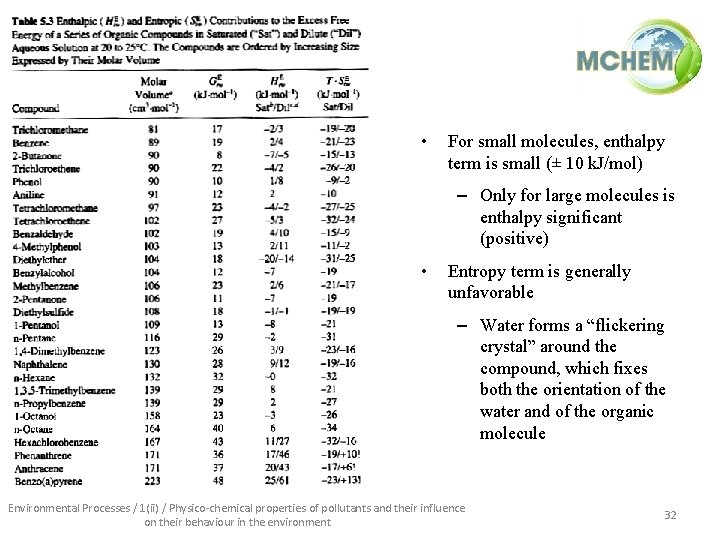
• For small molecules, enthalpy term is small (± 10 k. J/mol) – Only for large molecules is enthalpy significant (positive) • Entropy term is generally unfavorable – Water forms a “flickering crystal” around the compound, which fixes both the orientation of the water and of the organic molecule Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 32

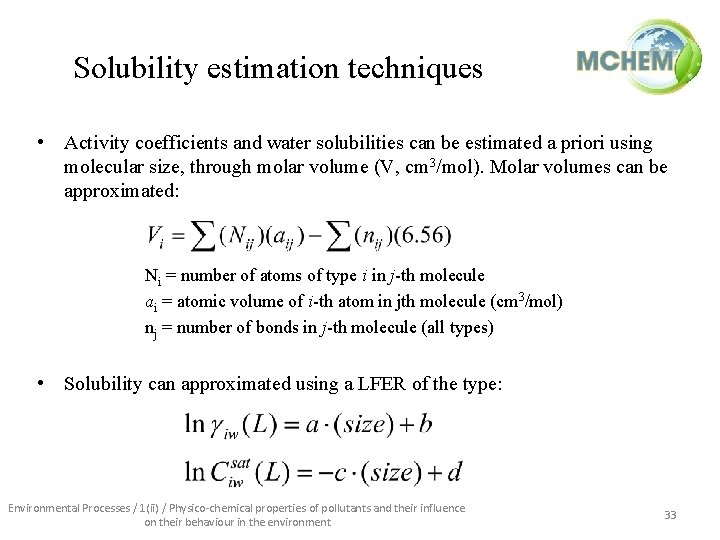
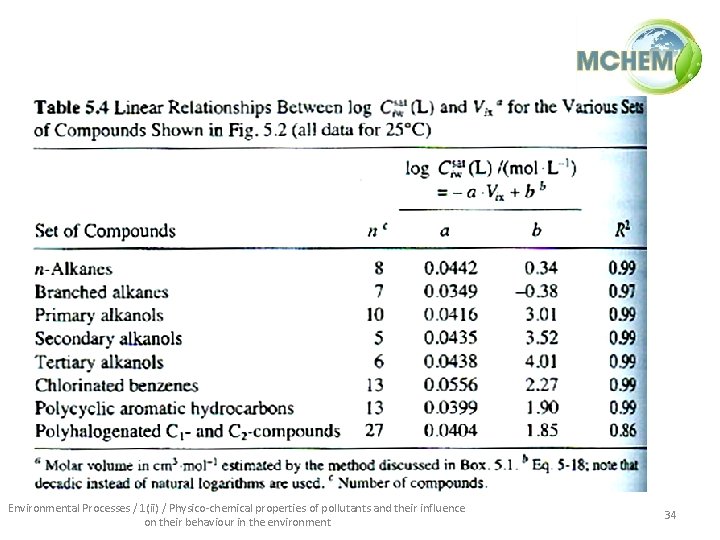
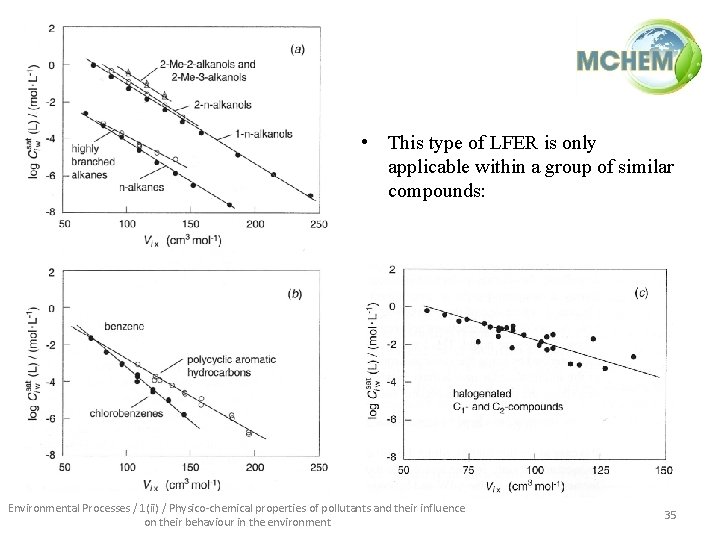
Solubility estimation techniques • Activity coefficients and water solubilities can be estimated a priori using molecular size, through molar volume (V, cm 3/mol). Molar volumes can be approximated: Ni = number of atoms of type i in j-th molecule ai = atomic volume of i-th atom in jth molecule (cm 3/mol) nj = number of bonds in j-th molecule (all types) • Solubility can approximated using a LFER of the type: Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 33

Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 34

• This type of LFER is only applicable within a group of similar compounds: Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 35

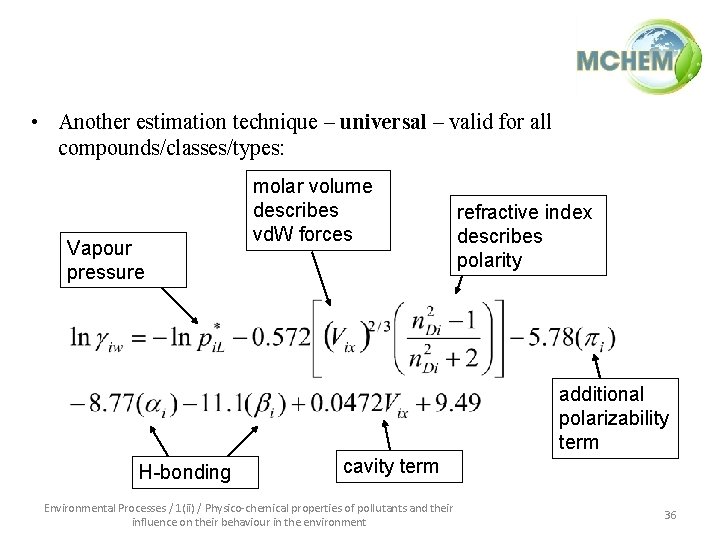
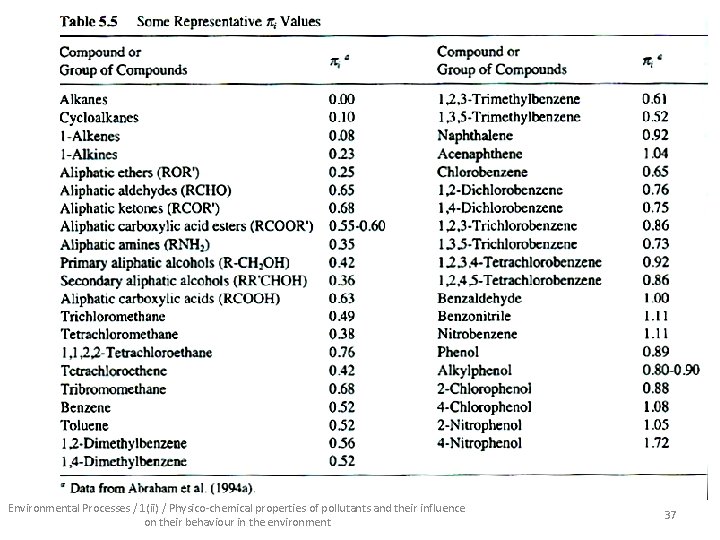
• Another estimation technique – universal – valid for all compounds/classes/types: Vapour pressure molar volume describes vd. W forces refractive index describes polarity additional polarizability term H-bonding cavity term Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 36

Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 37

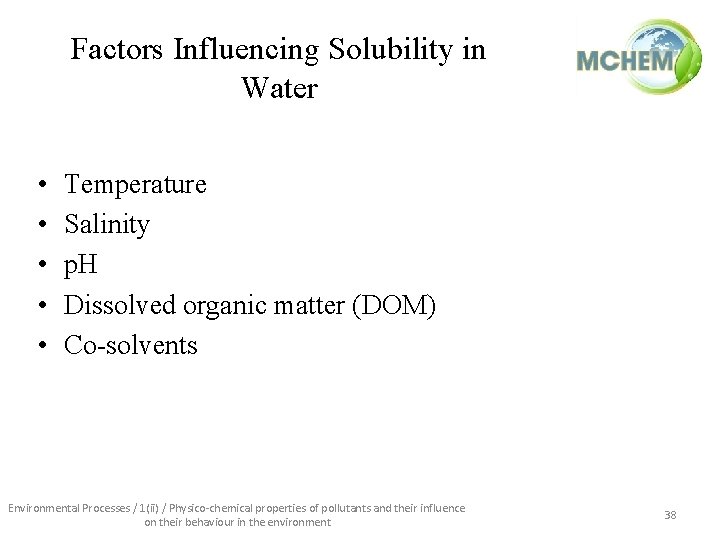
Factors Influencing Solubility in Water • • • Temperature Salinity p. H Dissolved organic matter (DOM) Co-solvents Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 38

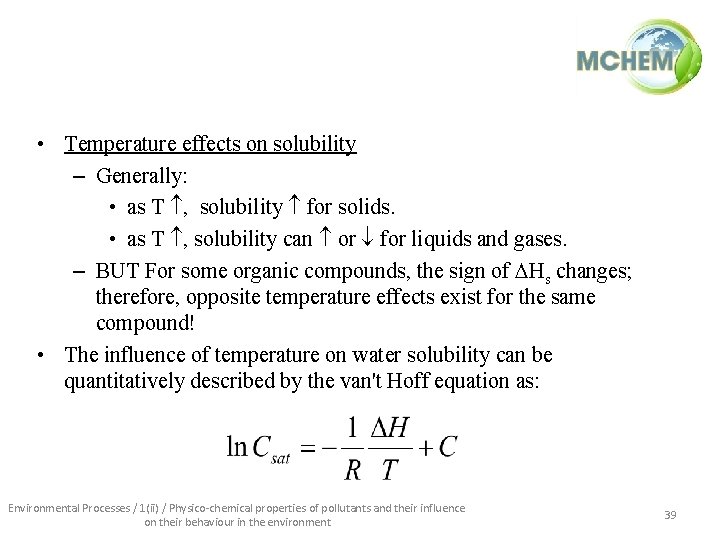
• Temperature effects on solubility – Generally: • as T , solubility for solids. • as T , solubility can or for liquids and gases. – BUT For some organic compounds, the sign of Hs changes; therefore, opposite temperature effects exist for the same compound! • The influence of temperature on water solubility can be quantitatively described by the van't Hoff equation as: Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 39

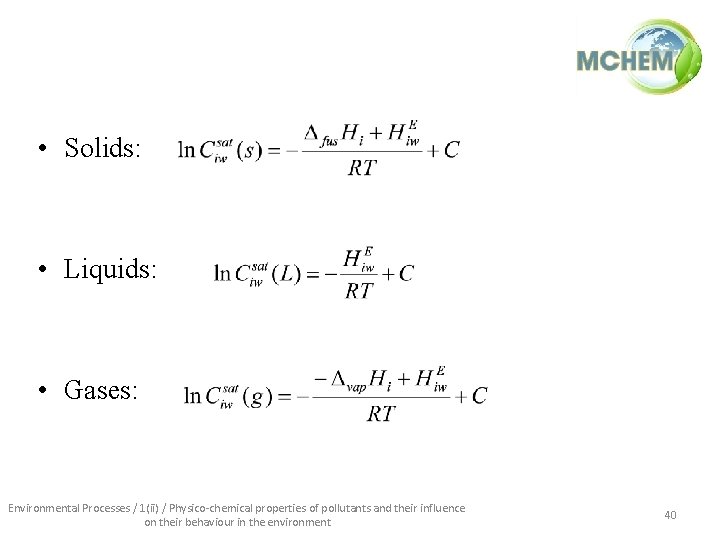
• Solids: • Liquids: • Gases: Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 40

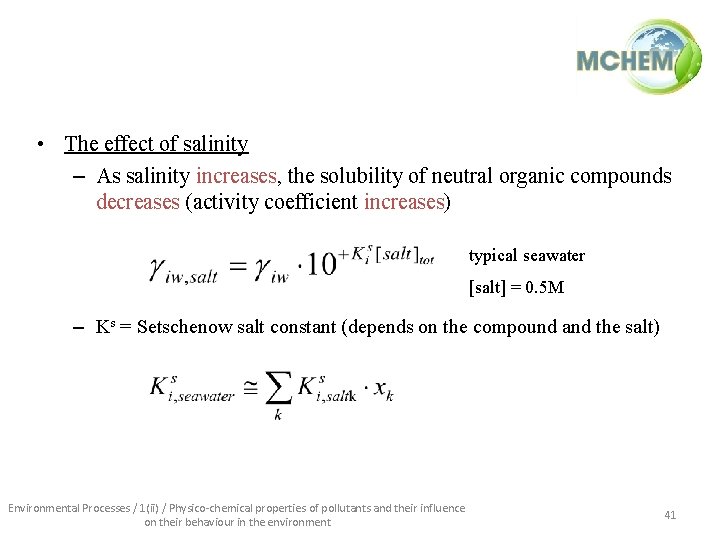
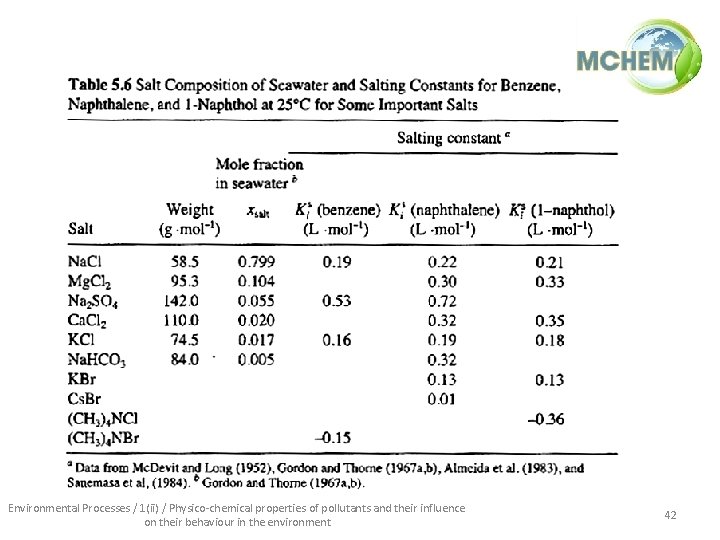
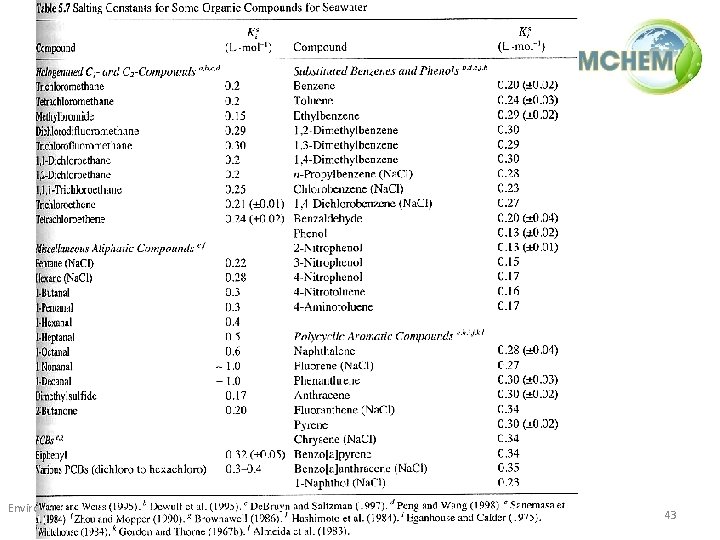
• The effect of salinity – As salinity increases, the solubility of neutral organic compounds decreases (activity coefficient increases) typical seawater [salt] = 0. 5 M – Ks = Setschenow salt constant (depends on the compound and the salt) Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 41

Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 42

Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 43

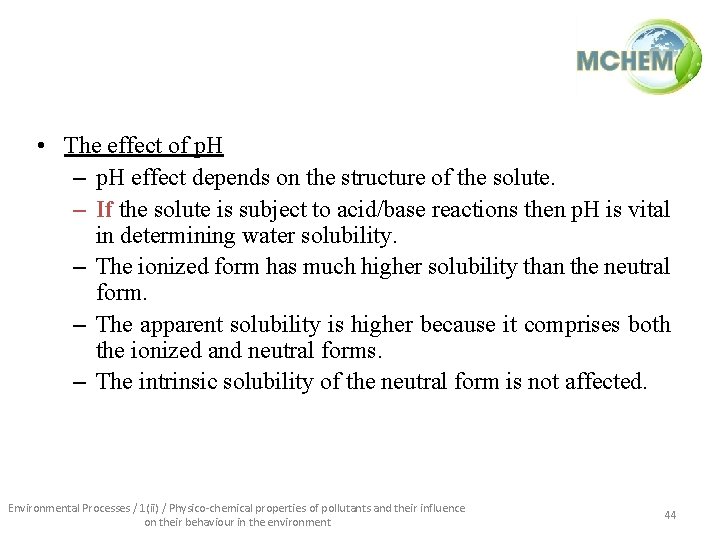
• The effect of p. H – p. H effect depends on the structure of the solute. – If the solute is subject to acid/base reactions then p. H is vital in determining water solubility. – The ionized form has much higher solubility than the neutral form. – The apparent solubility is higher because it comprises both the ionized and neutral forms. – The intrinsic solubility of the neutral form is not affected. Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 44

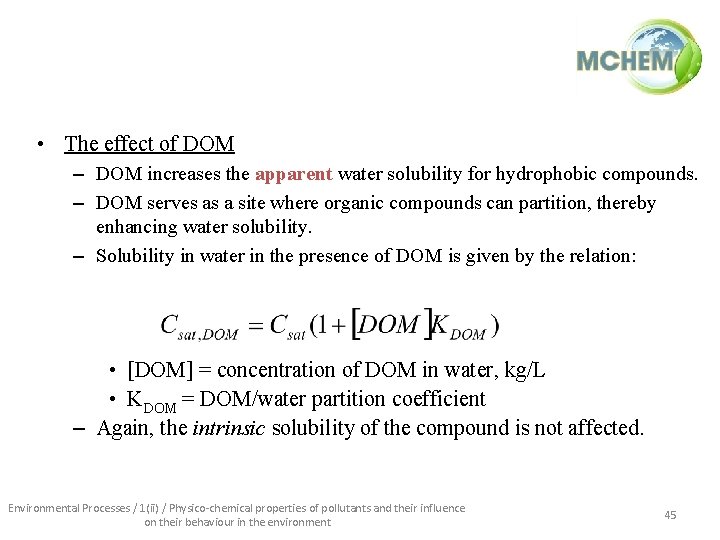
• The effect of DOM – DOM increases the apparent water solubility for hydrophobic compounds. – DOM serves as a site where organic compounds can partition, thereby enhancing water solubility. – Solubility in water in the presence of DOM is given by the relation: • [DOM] = concentration of DOM in water, kg/L • KDOM = DOM/water partition coefficient – Again, the intrinsic solubility of the compound is not affected. Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 45

• The effect of cosolvents – the presence of a co-solvent can increase the solubility of hydrophobic organic chemicals – co-solvents can completely change the solvation properties of “water” – examples: • industrial wastewaters • “gasohol” • engineered systems for soil or groundwater remediation • HPLC Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 46

• Solubility increases exponentially as cosolvent fraction increases. • Need 5 -10 volume % of cosolvent to see an effect. • Extent of solubility enhancement depends on type of cosolvent and solute: – effect is greatest for large, nonpolar solutes – more “organic” cosolvents have greater effect propanol>ethanol>methanol Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 47

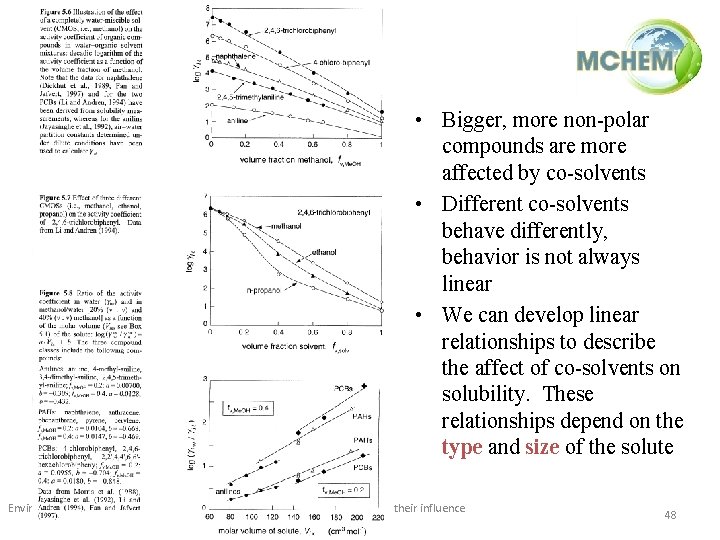
• Bigger, more non-polar compounds are more affected by co-solvents • Different co-solvents behave differently, behavior is not always linear • We can develop linear relationships to describe the affect of co-solvents on solubility. These relationships depend on the type and size of the solute Environmental Processes / 1(ii) / Physico-chemical properties of pollutants and their influence on their behaviour in the environment 48