Policy 108 OHSRP Quality Assurance and Quality Improvement











- Slides: 12

Policy 108 – OHSRP Quality Assurance and Quality Improvement Program OHSRP OFFICE OF POLICY AND ACCREDITATION

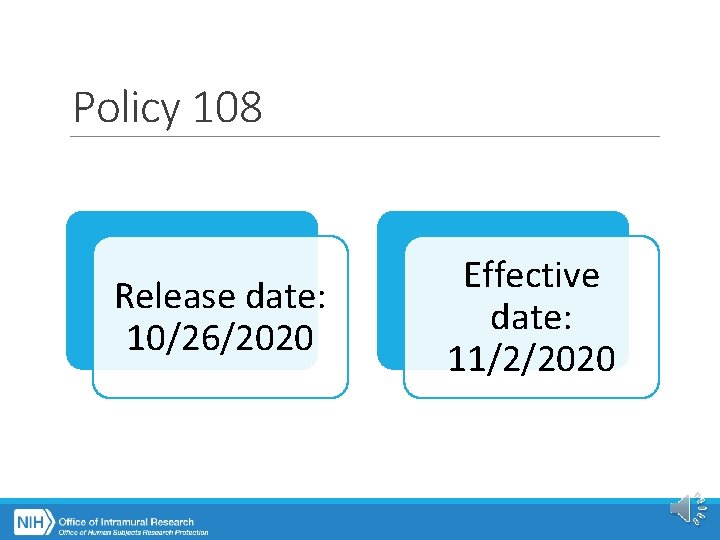
Policy 108 Release date: 10/26/2020 Effective date: 11/2/2020

Policy 108 – OHSRP Quality Assurance and Quality Improvement Program Purpose: • describe the OHSRP Quality Assurance and Quality Improvement Program (QA/QI Program) used to ensure that NIH IRB determinations are conducted in accordance with federal regulations and policy.

Policy 108 - Scope This policy applies to the OHSRP, including: • office of Compliance and Training • Office of IRB Operations (IRBO); and • The NIH Institutional Review Board (IRB)

Policy The OHSRP office of Compliance and Training (o. C&T) will conduct periodic quality assessments of the NIH IRB and the IRBO to ensure compliance with federal regulation and policy.

o. C&T Responsibilities The OHSRP o. C&T is responsible for QA/QI reviews of NIH IRB and IRBO activities, including: Conducting ongoing, periodic quality assessments to assess the effectiveness of the NIH IRB and the activities of the IRBO (see C. 1. above). These may include the following: • A random sampling of protocols; • A selection of studies using a risk-based approach; and/or • A review of documentation or observation of a meeting.

o. C&T Responsibilities (continued) • Conducting a directed QA/QI targeted or comprehensive review at the request of NIH HRPP leadership to provide an assessment of IRB compliance, and • Managing all activities related to the QA/QI program, including auditing IRB and IRBO activities using objective measures to assess quality and efficiency of the program.

Additional o. C&T Responsibilities Enhancing educational opportunities based on QA/QI review findings, provide ongoing education and assistance to NIH investigators, the IRB, and IRBO. Soliciting feedback from NIH investigators and Institute/Center leadership regarding the IRB and IRBO activities, and encouraging NIH investigators to communicate concerns or suggestions.

Executive Chair and IRBO Director Responsibility Are responsible for providing timely written responses to QA/QI review findings regarding the IRB or the IRBO, respectively.

OHSRP and Institutional Official (IO) Responsibilities The Director of the OHSRP and the Institutional Official are responsible for reviewing QA/QI findings and taking appropriate actions to remediate any identified noncompliance.

Do Communicate concerns or suggestions to OHSRP regarding the NIH IRB or IRBO. What is expected by OHSRP? to provide feedback to Don’t Forget QAQI surveys

Resources Policy 108 and associated change table and guidance is posted on the IRBO website Questions? Contact IRB@od. nih. gov
 Total quality management seminar
Total quality management seminar Quality improvement vs quality assurance
Quality improvement vs quality assurance Obtuse
Obtuse Fvad formula
Fvad formula Ohsrp
Ohsrp Perform quality assurance
Perform quality assurance Qa basic concepts
Qa basic concepts Pmp quality management
Pmp quality management Pmp gold plating
Pmp gold plating Software testing and quality assurance: theory and practice
Software testing and quality assurance: theory and practice Quality revolution
Quality revolution Software testing and quality assurance theory and practice
Software testing and quality assurance theory and practice Software testing and quality assurance theory and practice
Software testing and quality assurance theory and practice