Polarity What is electronegativity Review Bond Types Ionic

Polarity

What is electronegativity?

Review: Bond Types Ionic – transfer of electrons – EN difference of greater than 1. 7

Review: Bond Types Covalent – sharing of electrons – – Nonpolar: electrons shared evenly ● Two of the same atoms bonded together ● EN difference of close to 0 (less than 0. 4) Polar: electrons shared unevenly ● EN difference of less than 1. 7 ● More EN element attracts electrons more

Polar vs. Nonpolar Bonds CO F 2

Polar vs. Nonpolar Bonds H 2 O N 2

Which bond is more polar? ● C-O or C-F ? ● Al-Cl or Br-Br ? ● S-Cl or Si-H ? ● H-Se or H-Te ?

Polarity of Molecules It's all about the symmetry ● If the molecule is symmetrical all the way around, it is nonpolar ● If it is asymmetrical (uneven) in any way, it is polar ●

Polarity of Molecules SNAP Symmetrical = Nonpolar, Asymmetrical = Polar

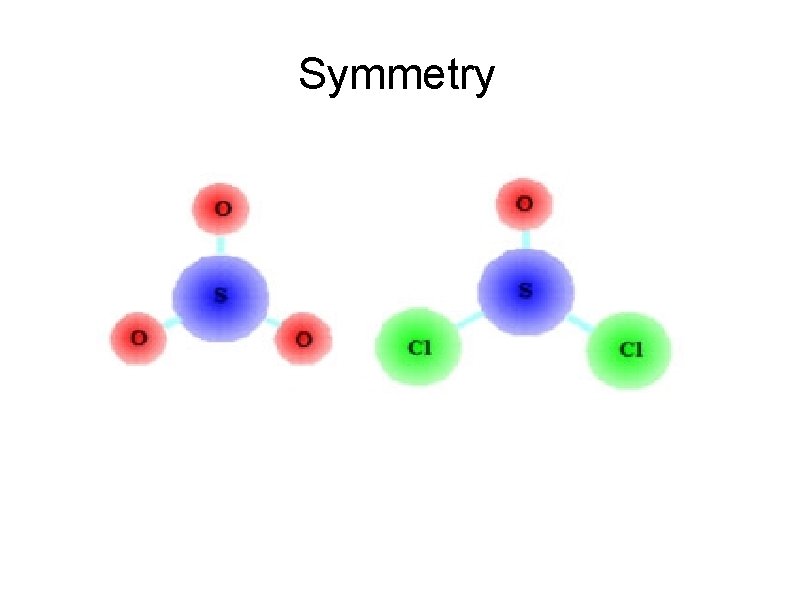
Symmetry

WATER IS POLAR

CH 4 CH 3 Br CH 2 Br 2

Al. Cl 3

O 2

H 2 Te

CO For each: 1) Draw Lewis structure 2) Are bonds polar or nonpolar? 3) Is molecule polar or nonpolar? CO 2

Polarity of Bonds and Molecules ● Bond polarity: look at EN difference (END) ● Molecule polarity: look at symmetry ● A nonpolar molecule can have polar bonds ● A polar molecule can have nonpolar bonds, but this is rare (example: ozone)
- Slides: 18