Polarity Bonds and Molecules What holds molecules together
Polarity Bonds and Molecules

What holds molecules together? Bonds are made up of? Electrons How do the electrons hold atoms together?
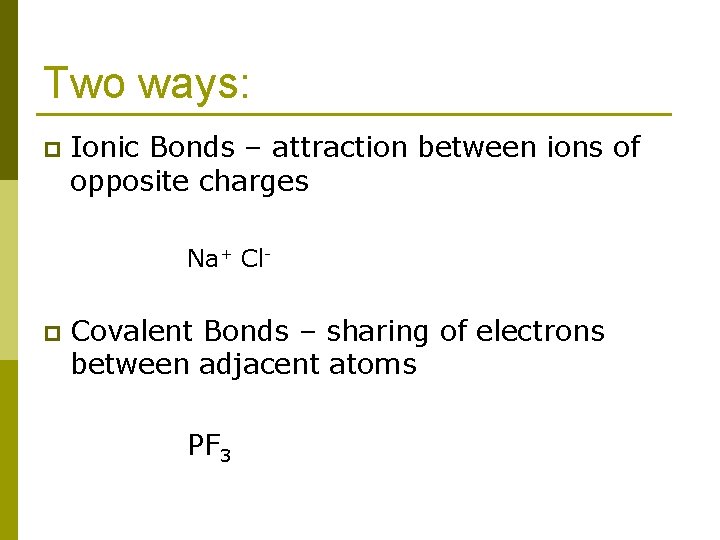
Two ways: p Ionic Bonds – attraction between ions of opposite charges Na+ Cl- p Covalent Bonds – sharing of electrons between adjacent atoms PF 3
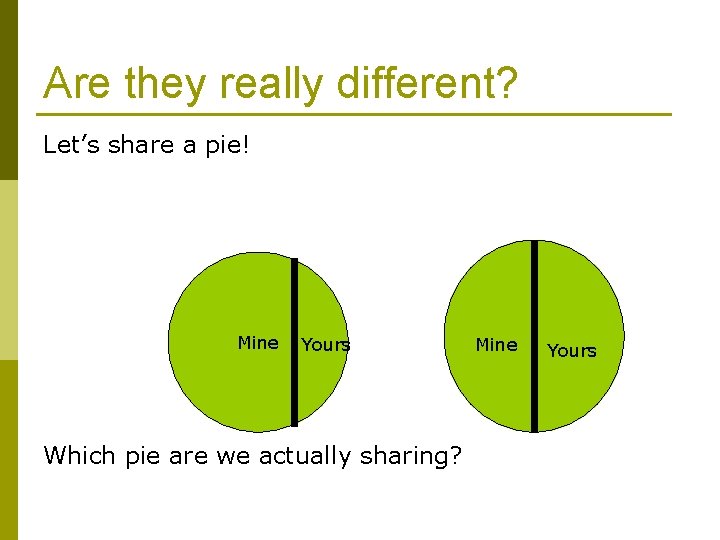
Are they really different? Let’s share a pie! Mine Yours Which pie are we actually sharing? Mine Yours
Sharing doesn’t have to be equal! Mine
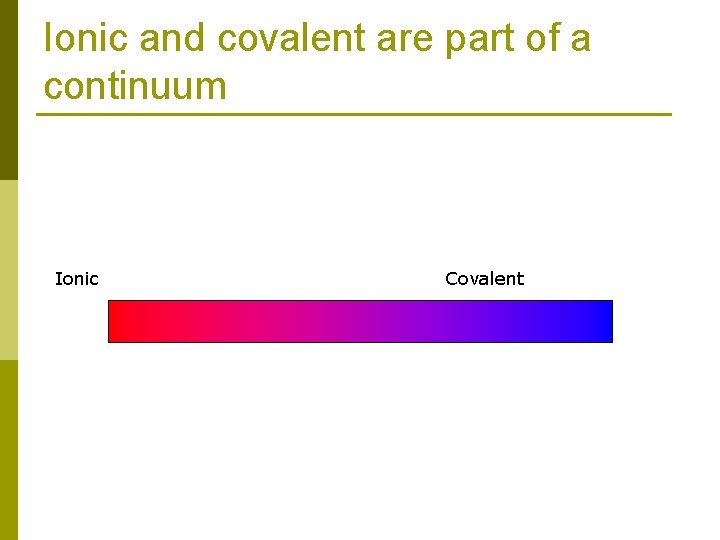
Ionic and covalent are part of a continuum Ionic Covalent
Two extremes Mine Yours Ours
Something in the middle Mine Yours
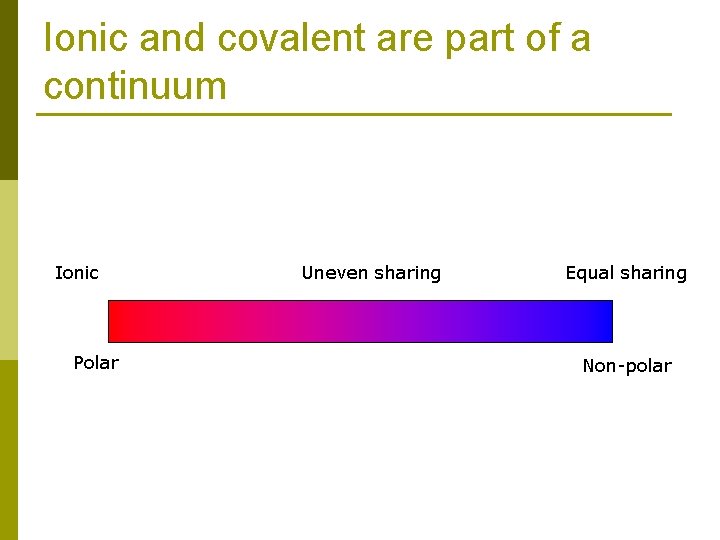
Ionic and covalent are part of a continuum Ionic Polar Uneven sharing Equal sharing Non-polar

So, consider a bond, any bond: Cl – Cl Which case is this? Equal sharing!

So, consider a bond, any bond: H-Cl Which case is this? Unequal sharing! How do you know? They are on opposite sides of the Periodic table!
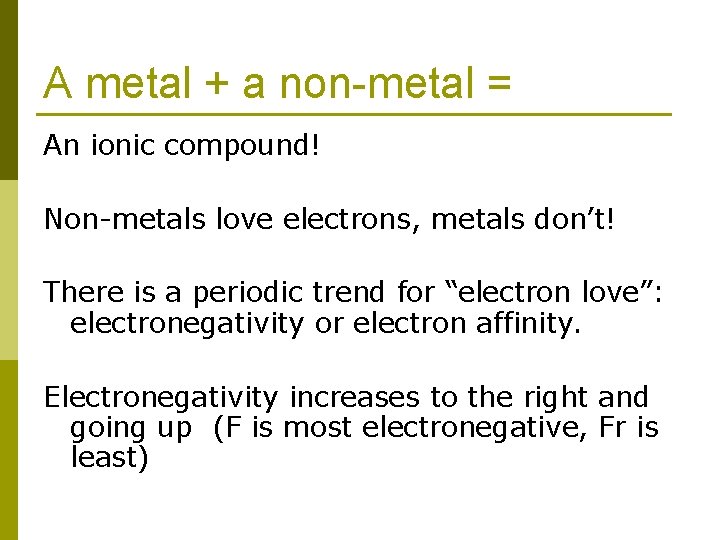
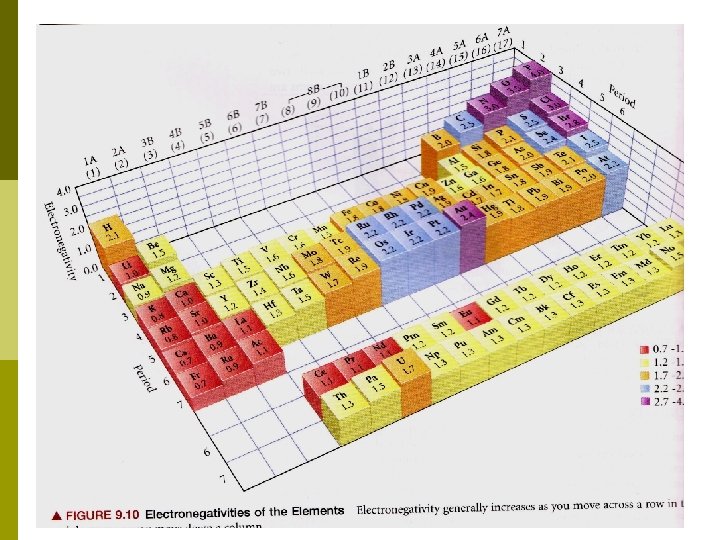
A metal + a non-metal = An ionic compound! Non-metals love electrons, metals don’t! There is a periodic trend for “electron love”: electronegativity or electron affinity. Electronegativity increases to the right and going up (F is most electronegative, Fr is least)
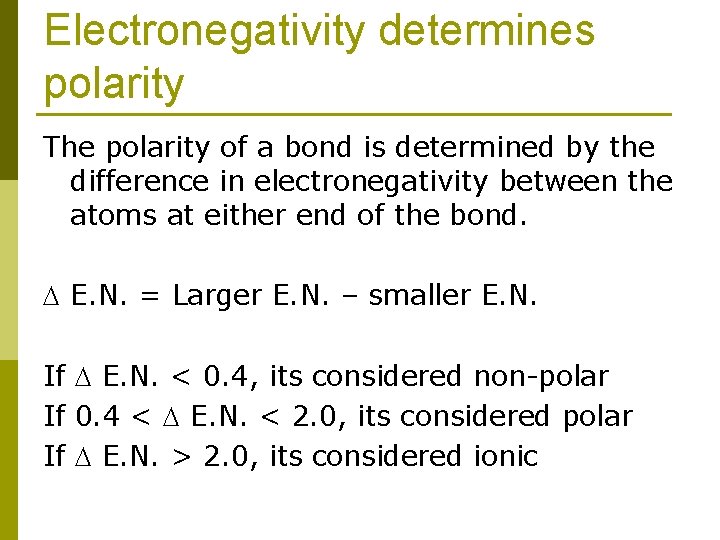
Electronegativity determines polarity The polarity of a bond is determined by the difference in electronegativity between the atoms at either end of the bond. E. N. = Larger E. N. – smaller E. N. If E. N. < 0. 4, its considered non-polar If 0. 4 < E. N. < 2. 0, its considered polar If E. N. > 2. 0, its considered ionic
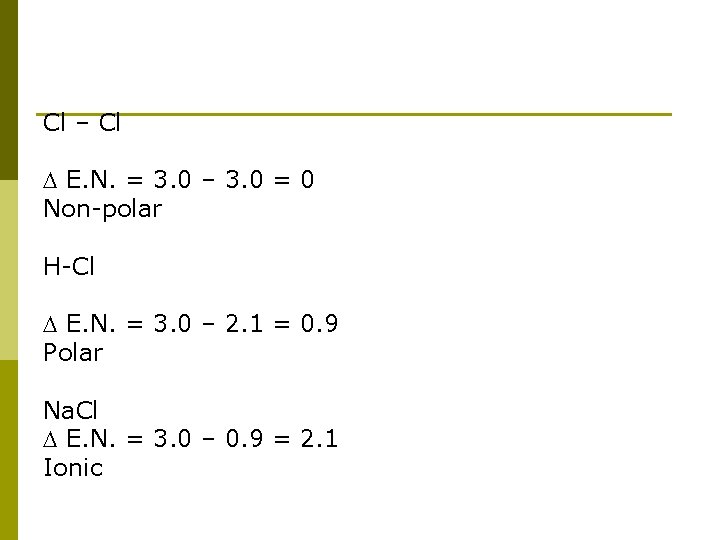
Cl – Cl E. N. = 3. 0 – 3. 0 = 0 Non-polar H-Cl E. N. = 3. 0 – 2. 1 = 0. 9 Polar Na. Cl E. N. = 3. 0 – 0. 9 = 2. 1 Ionic

Polarity is represented as an arrow… …pointing toward the more negative atom. Cl – Cl H-Cl Na. Cl
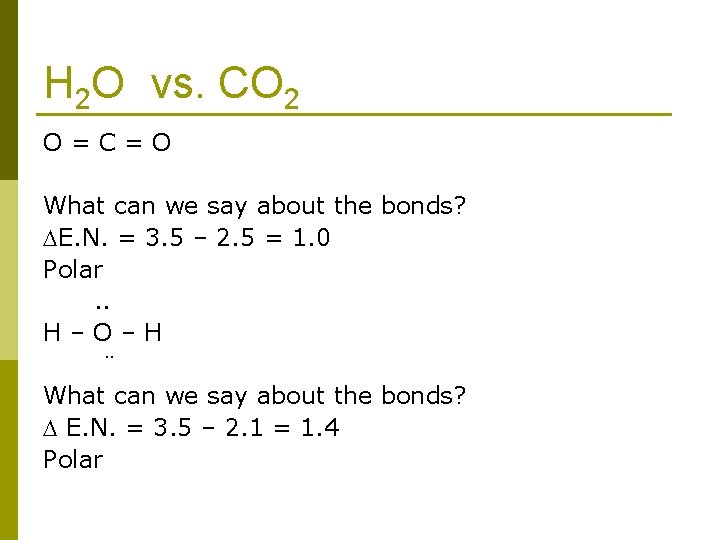
H 2 O vs. CO 2 O=C=O What can we say about the bonds? E. N. = 3. 5 – 2. 5 = 1. 0 Polar. . H–O–H ¨ What can we say about the bonds? E. N. = 3. 5 – 2. 1 = 1. 4 Polar

Does that make sense? What do you know about water? Yup, it’s a polar solvent. What about CO 2? Would you expect it to be polar? Would it surprise you to learn that CO 2 is NONPOLAR? !? !? !?
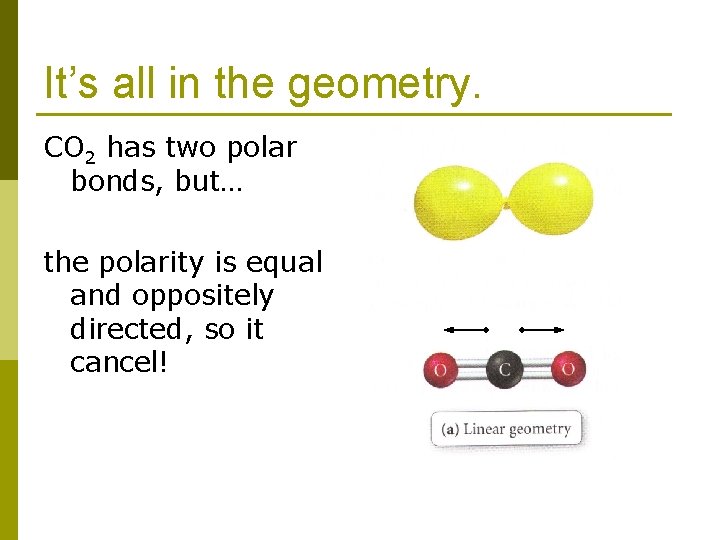
It’s all in the geometry. CO 2 has two polar bonds, but… the polarity is equal and oppositely directed, so it cancel!

H 2 O Water also has two equally polar bonds, but they aren’t pointing in opposite directions.
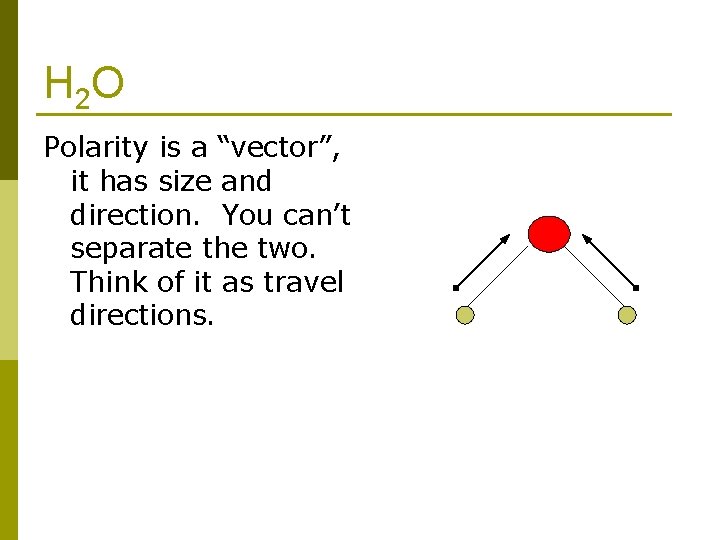
H 2 O Polarity is a “vector”, it has size and direction. You can’t separate the two. Think of it as travel directions.
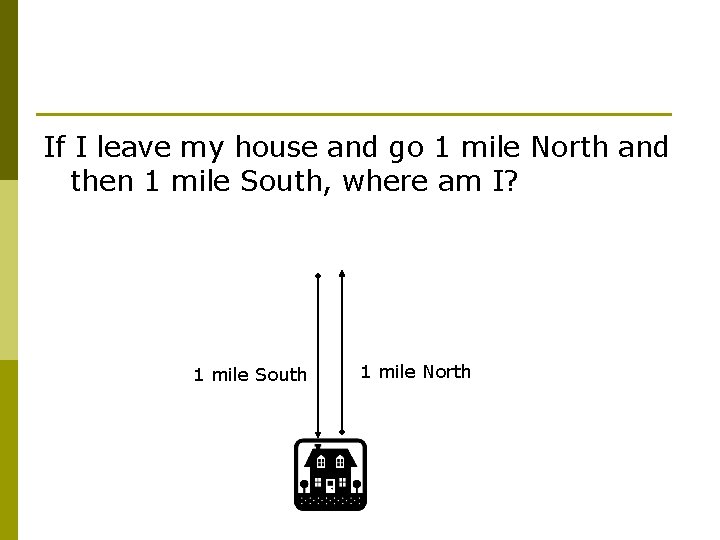
If I leave my house and go 1 mile North and then 1 mile South, where am I? 1 mile South 1 mile North
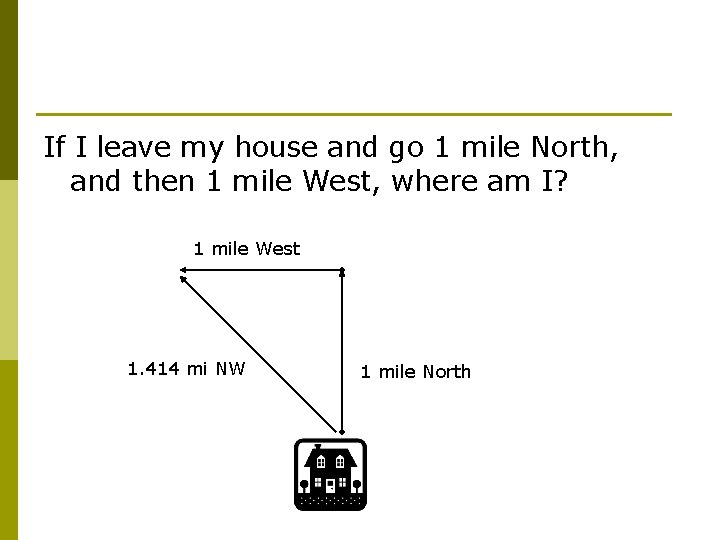
If I leave my house and go 1 mile North, and then 1 mile West, where am I? 1 mile West 1. 414 mi NW 1 mile North
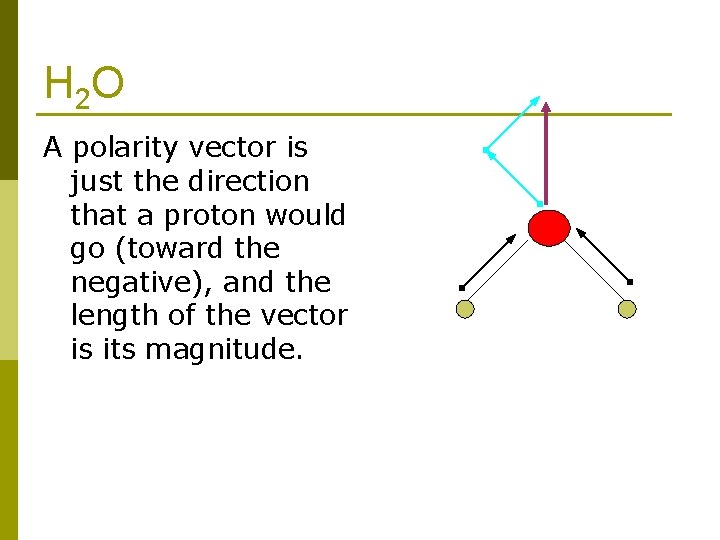
H 2 O A polarity vector is just the direction that a proton would go (toward the negative), and the length of the vector is its magnitude.
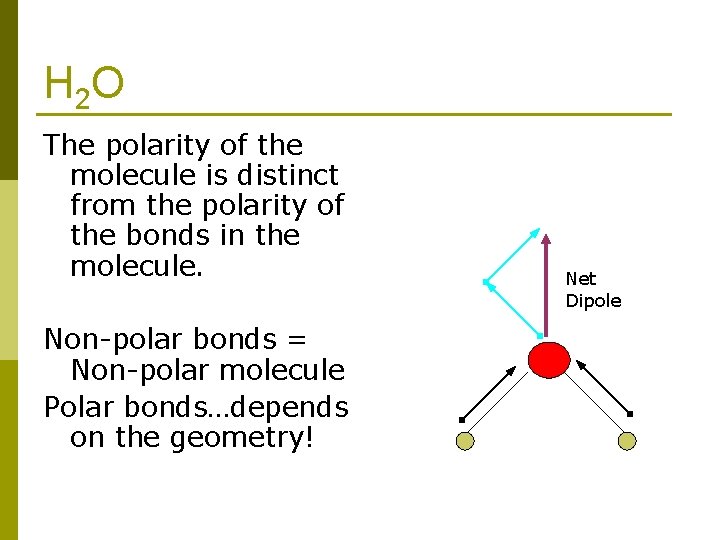
H 2 O The polarity of the molecule is distinct from the polarity of the bonds in the molecule. Non-polar bonds = Non-polar molecule Polar bonds…depends on the geometry! Net Dipole

Clicker Is CCl 4… A. B. C. Polar Non-polar Ionic
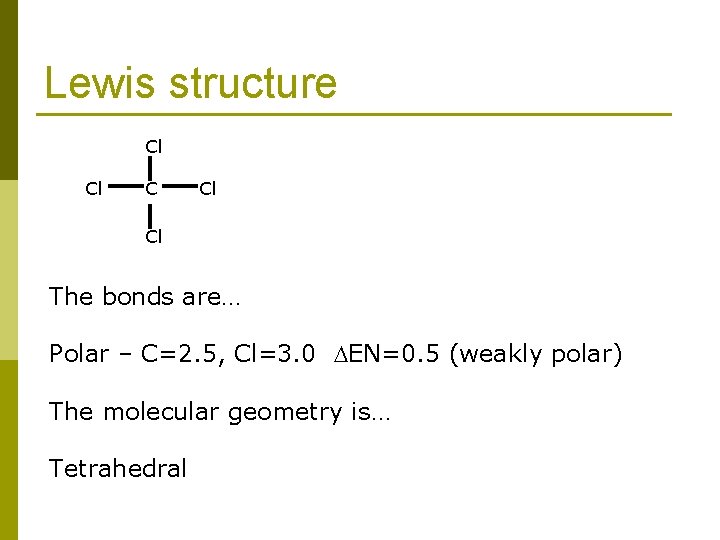
Lewis structure Cl Cl Cl The bonds are… Polar – C=2. 5, Cl=3. 0 EN=0. 5 (weakly polar) The molecular geometry is… Tetrahedral
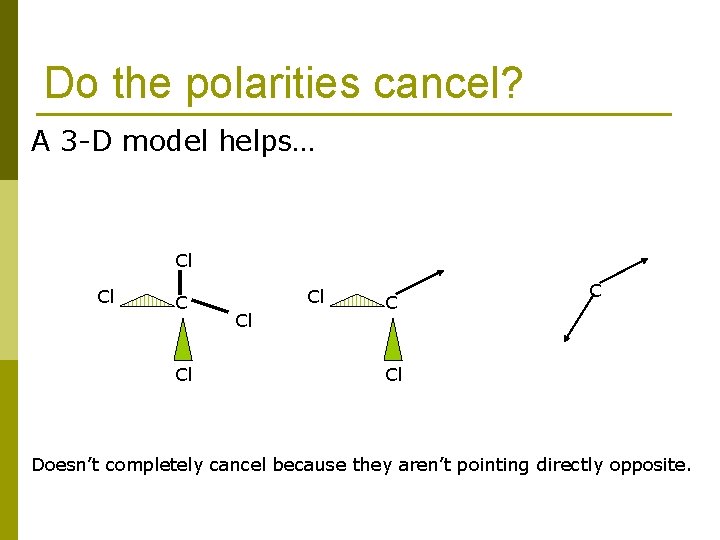
Do the polarities cancel? A 3 -D model helps… Cl Cl Cl C C Cl Doesn’t completely cancel because they aren’t pointing directly opposite.

Clicker Is NH 3… A. B. C. Polar Non-polar Ionic
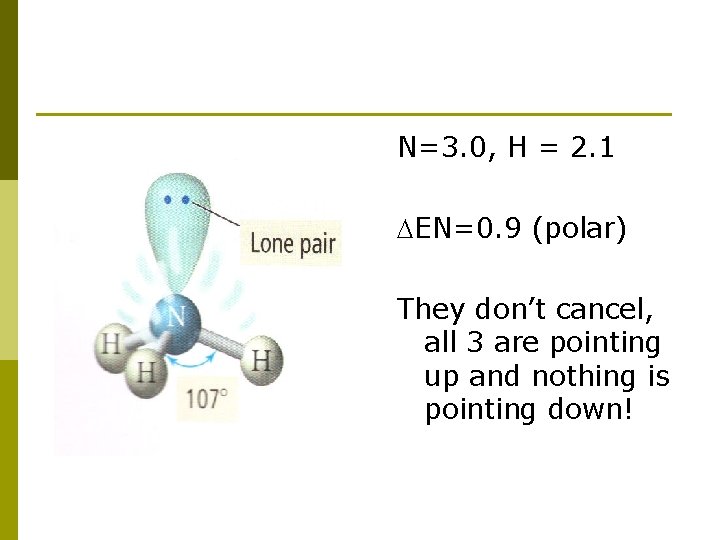
N=3. 0, H = 2. 1 EN=0. 9 (polar) They don’t cancel, all 3 are pointing up and nothing is pointing down!

Clicker Is HCN… A. B. C. Polar Non-polar Ionic
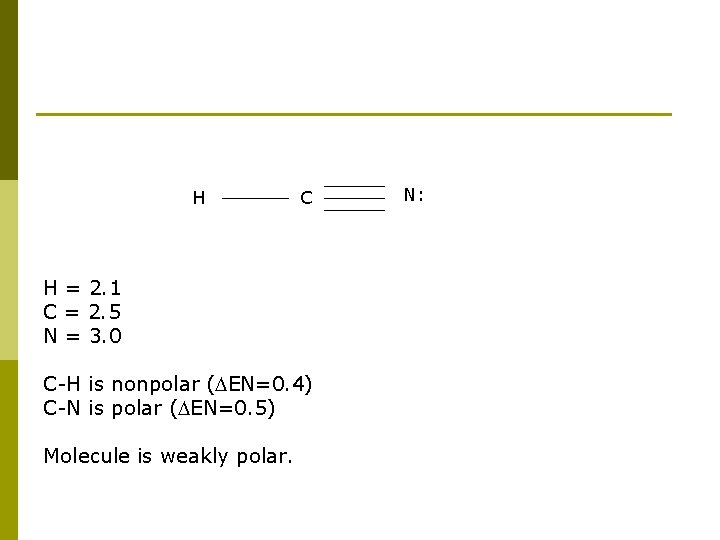
H C H = 2. 1 C = 2. 5 N = 3. 0 C-H is nonpolar ( EN=0. 4) C-N is polar ( EN=0. 5) Molecule is weakly polar. N:

Hybridization… What do S and P orbitals really look like?
- Slides: 34