POLARITY AND SOLUBILITY Like dissolves like 1 a

POLARITY AND SOLUBILITY: “Like dissolves like”

1. a) Review of shapes: What are the five basic shapes? § Linear § Trigonal Planar § Tetrahedral § Trigonal Pyramidal § Bent

1. b) Review of Bonding: Ionic or covalent? § Ionic = metal + nonmetal § Covalent = nonmetal + nonmetal § Nonpolar Covalent = electrons shared equally § Polar Covalent = electrons shared unequally

2. Definitions of Polarity § a)Polar bond— bond involves unequal distribution of electrons § b)Polar molecule— the molecule has an unequal distribution of electrons § c)Dipole—charges in a molecule are separated; use and
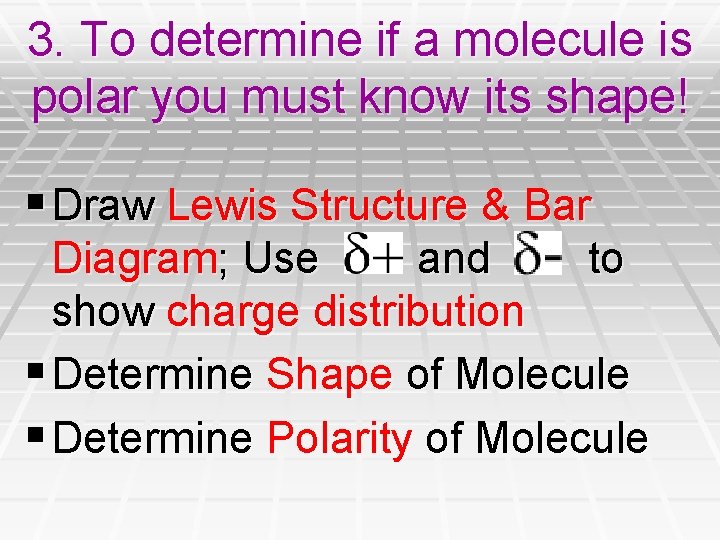
3. To determine if a molecule is polar you must know its shape! § Draw Lewis Structure & Bar Diagram; Use and to show charge distribution § Determine Shape of Molecule § Determine Polarity of Molecule
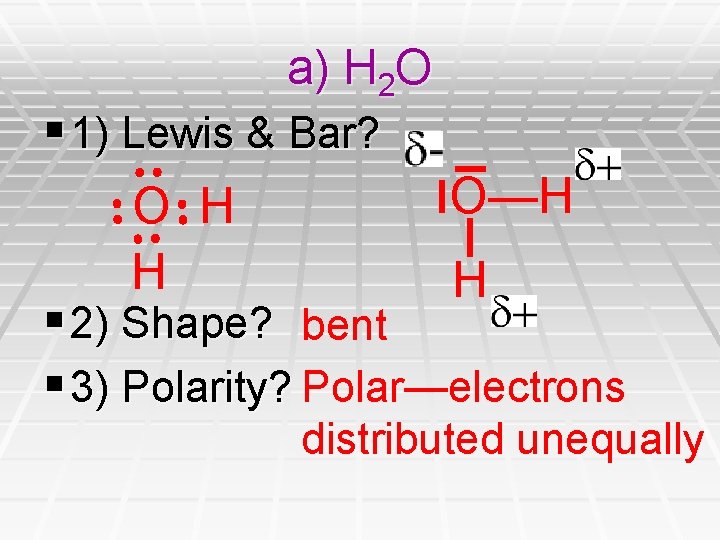
a) H 2 O § 1) Lewis & Bar? O—H O H H H § 2) Shape? bent § 3) Polarity? Polar—electrons distributed unequally
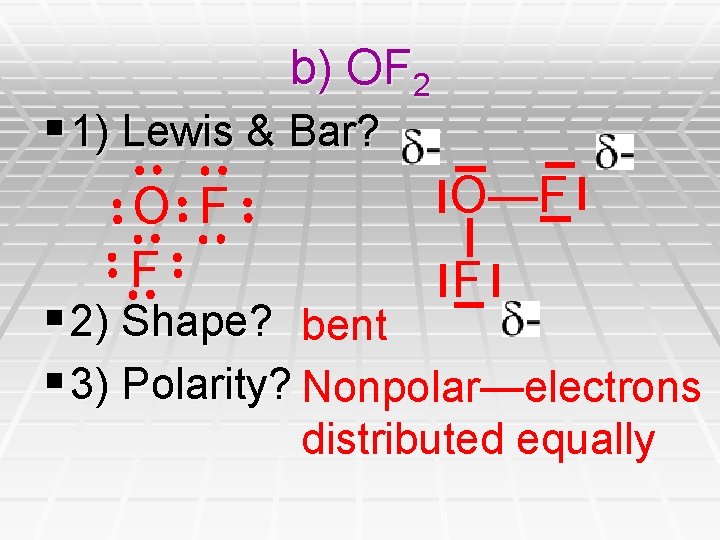
b) OF 2 § 1) Lewis & Bar? O—F O F F F § 2) Shape? bent § 3) Polarity? Nonpolar—electrons distributed equally
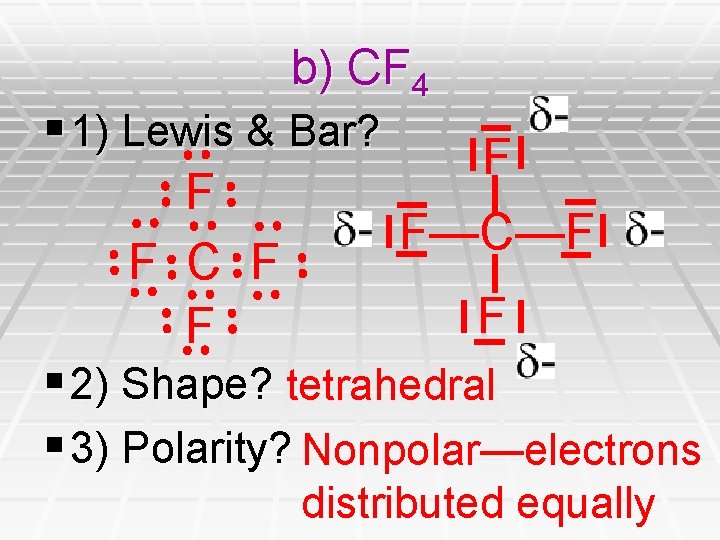
b) CF 4 § 1) Lewis & Bar? F F F—C—F F C F F F § 2) Shape? tetrahedral § 3) Polarity? Nonpolar—electrons distributed equally

4. Determining solubility: “Like Dissolves Like” § Polar compounds like water dissolve polar and ionic solids like Na. Cl § Nonpolar compounds like oils and fats dissolve other nonpolar compounds

5. Like Dissolves Like: Why is this important? § Many pesticides and other dangerous toxins are nonpolar and therefore fat soluble § These nonpolar substances build up in the fat reserves of animals, causing harm § DDT in eagles; PCB’s in humans

Determining Solubility Examples: 6. a) Are these pairs soluble? § HCl H—Cl H 2 O O—H H § Both are polar, therefore they are soluble

Determining Solubility Examples: 6. b) Are these pairs soluble? § CF 4 F F—C—F NH 3 H—N—H H F § CF 4 is nonpolar, NH 3 is polar; therefore NOT SOLUBLE

7. Practical Application: Chromatography § Pens can be identified using chromatography § Pen ink is a mixture which can be separated using various solvents § A chromatogram (“color picture”) results from the process of chromatography
- Slides: 13