Polar vs NonPolar Molecules Polarity in a molecules













- Slides: 15

Polar vs. Non-Polar Molecules Polarity in a molecules determines whether or not electrons in that molecule are shared equally. (Electronegativity) When determining the polarity of a molecule, it is all about symmetry. Asymmetric molecules tend to be polar. Symmetric molecules are always nonpolar.

When determining the polarity of a molecule, follow these steps: Draw the Electron Dot structure of the molecule. Determine the VSEPR geometry Decide if the molecule is symmetric or not. – this is by looking at the VSEPR geometry – polarity (dipole moments) within the individual bonds

Dipole Moment Direction of the polar bond in a molecule. Arrow points toward the more e-neg atom. + H Cl

The molecule is non-polar if : each bond in the molecule is non-polar and there are no unbonded electron pairs. each bond in the molecule has the same polarity and there are no unbonded electron pairs on the central atom. There is no net dipole moment (all moments cancel out)

Nonpolar Molecules Dipole moments are symmetrical and cancel out. F BF 3 B F F

The molecule is polar if: There is a net dipole moment each bond in the molecule is non-polar, but there are unbonded electron pairs on the central atom. bonds in the molecule have different polarities and/or there are unbonded electron pairs on the central atom.

Polar Molecules – Dipole moments are asymmetrical and don’t cancel. – Molecule has a net dipole moment. O H 2 O H H net dipole moment

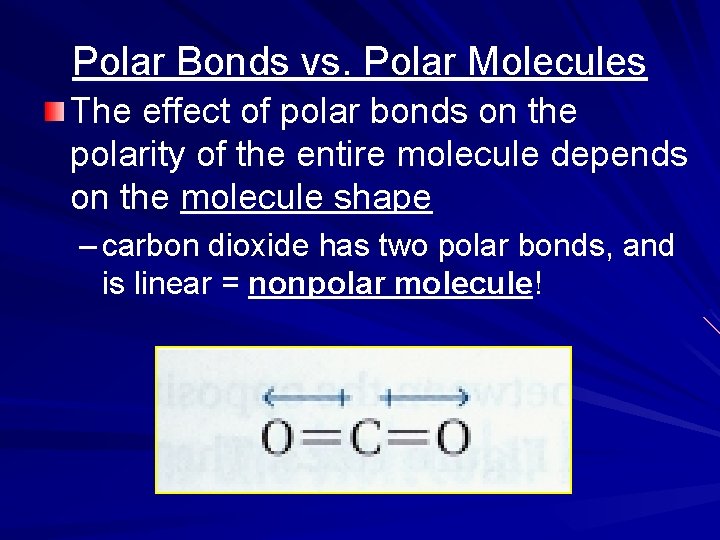
Polar Bonds vs. Polar Molecules The effect of polar bonds on the polarity of the entire molecule depends on the molecule shape – carbon dioxide has two polar bonds, and is linear = nonpolar molecule!

Molecular Dipole Moments - O + C O - • Carbon dioxide has no dipole moment • The bonds are polar covalent • VSEPR shape is linear • because individual bond dipoles cancel each other out. – They are equal and opposite

Determining Molecular Polarity Depends on: – dipole moments – molecular shape

Polar molecules The effect of polar bonds on the polarity of the entire molecule depends on the molecule shape – water has two polar bonds and a bent shape; the highly electronegative oxygen pulls the eaway from H = very polar!

Determining Molecular Polarity Therefore, polar molecules have. . . – asymmetrical shape (lone pairs) or – asymmetrical atoms H CHCl 3 Cl Cl Cl net dipole moment

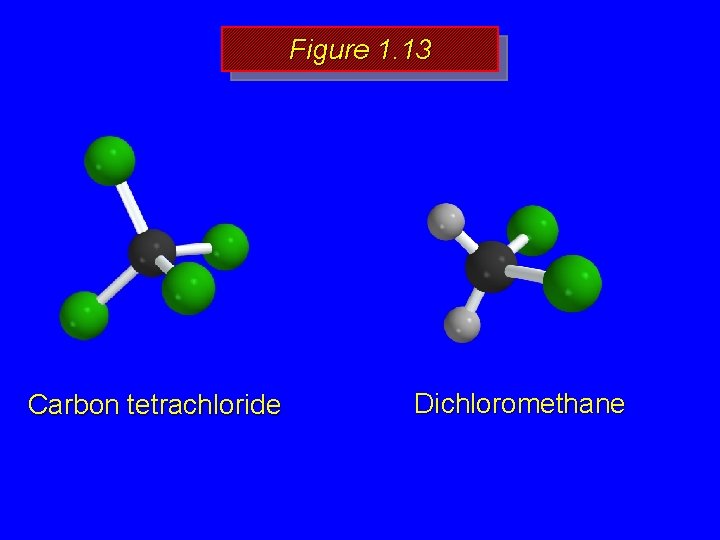
Figure 1. 13 Carbon tetrachloride Dichloromethane

Figure 1. 13 Resultant of these two bond dipoles is Carbon tetrachloride has no dipole moment because all of the individual bond dipoles cancel.

Figure 1. 13 Resultant of these two bond dipoles is The individual bond dipoles do not cancel in dichloromethane; it has a dipole moment.