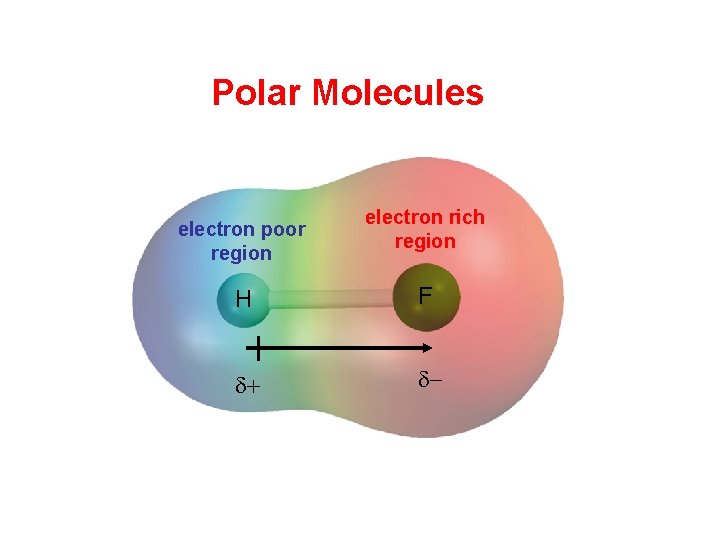
Polar Molecules electron poor region electron rich region




- Slides: 20

Polar Molecules electron poor region electron rich region H F d+ d-

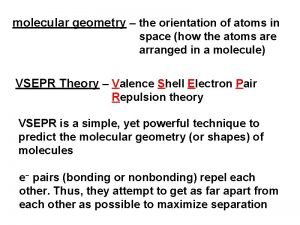
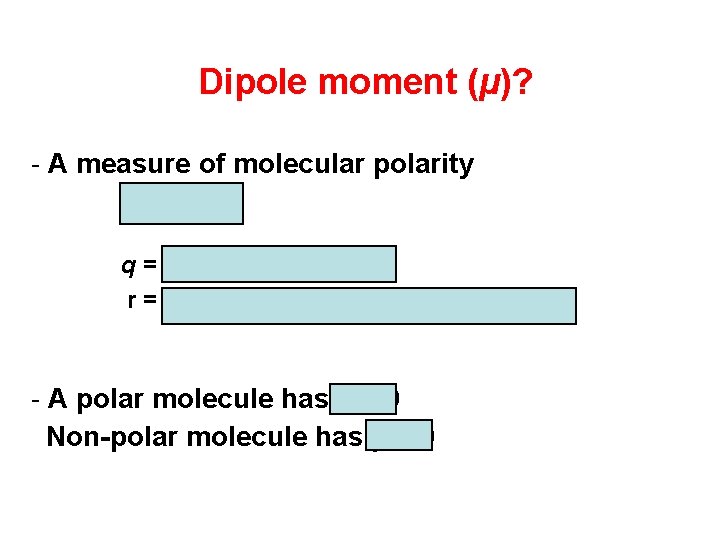
Dipole moment (µ)? - A measure of molecular polarity m=qxr q = charge (coulomb) r = the distance between charges (m) - A polar molecule has µ ≠ 0 Non-polar molecule has µ = 0

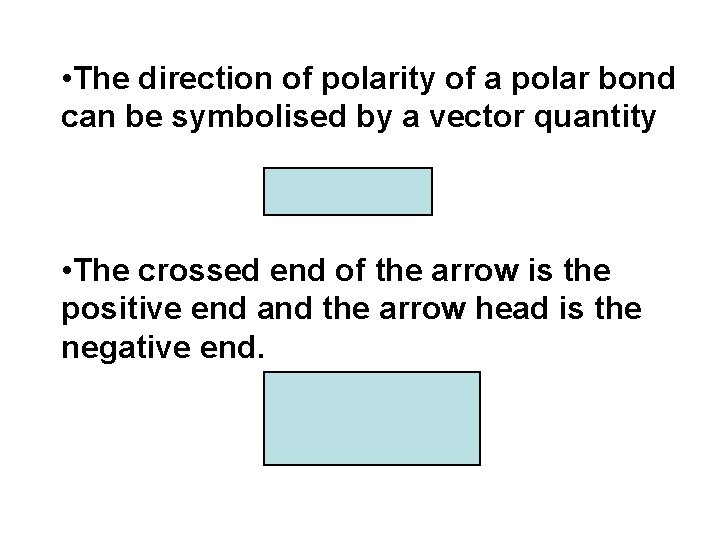
• The direction of polarity of a polar bond can be symbolised by a vector quantity ( ) • The crossed end of the arrow is the positive end and the arrow head is the negative end. H Cl

Example:

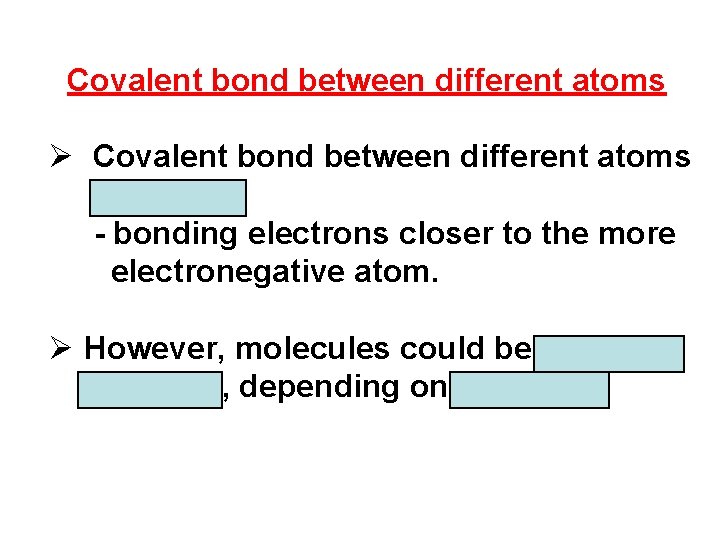
Covalent bond between same atoms Ø Covalent bond between atoms of the same element is nonpolar → nonpolar molecule. Ø Bonding electrons are shared equally. Example:

Covalent bond between different atoms Ø Covalent bond between different atoms is polar. - bonding electrons closer to the more electronegative atom. Ø However, molecules could be polar or nonpolar, depending on its shape.

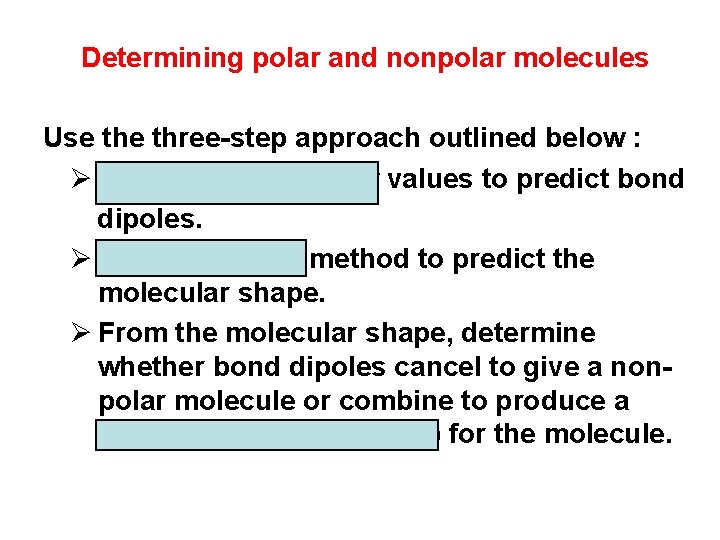
Determining polar and nonpolar molecules Use three-step approach outlined below : Ø Use electronegativity values to predict bond dipoles. Ø Use the VSEPR method to predict the molecular shape. Ø From the molecular shape, determine whether bond dipoles cancel to give a nonpolar molecule or combine to produce a (resultant dipole moment) for the molecule.

Molecules with different atoms & asymmetrically arranged are polar. Example:

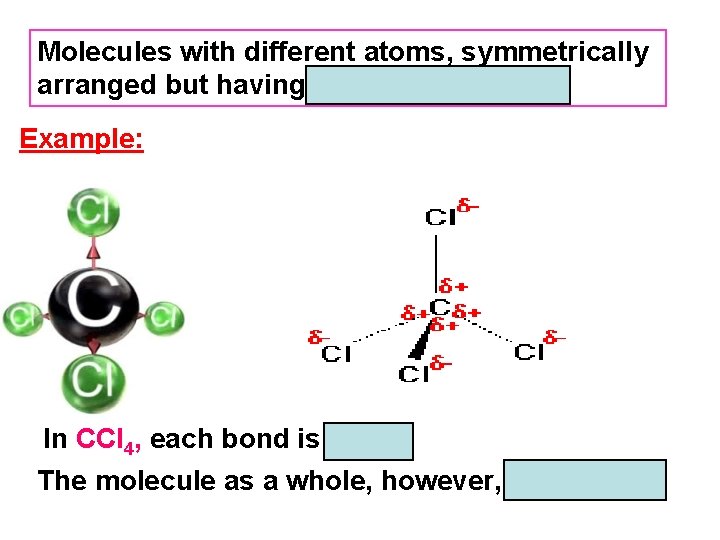
Molecules with different atoms, symmetrically arranged but having µ = 0 are nonpolar. Example: In CCl 4, each bond is polar. The molecule as a whole, however, is nonpolar

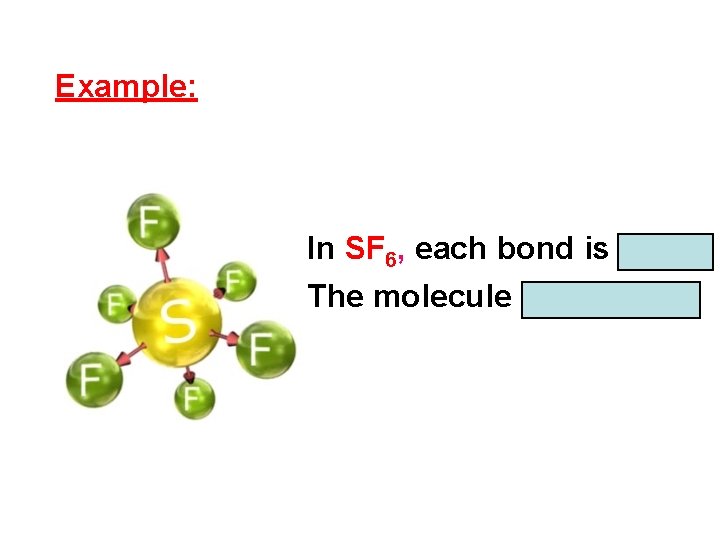
Example: In SF 6, each bond is polar. The molecule is nonpolar

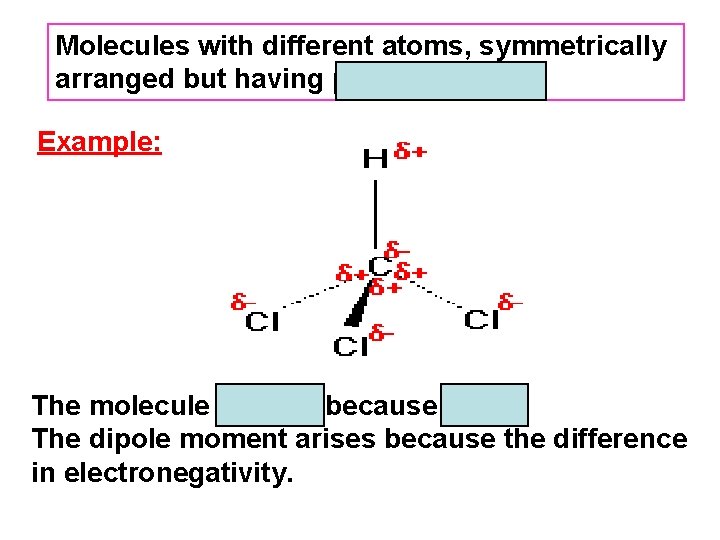
Molecules with different atoms, symmetrically arranged but having µ ≠ 0 are polar. Example: The molecule is polar because µ ≠ 0. The dipole moment arises because the difference in electronegativity.

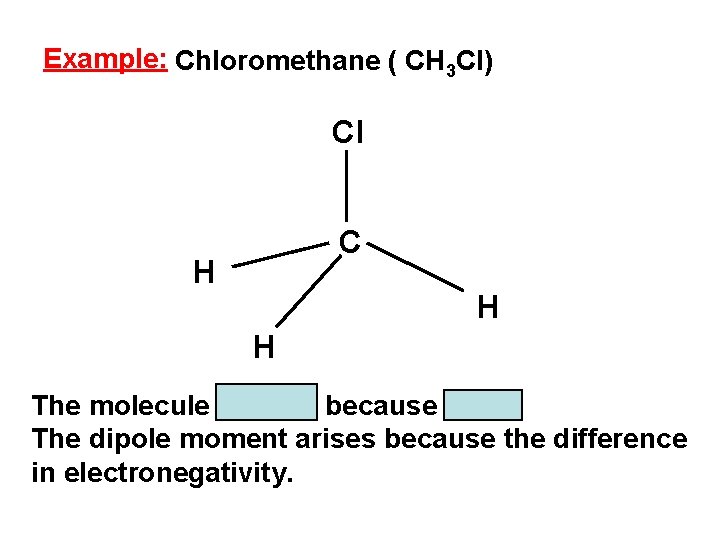
Example: Chloromethane ( CH 3 Cl) Cl C H H H The molecule is polar because µ ≠ 0. The dipole moment arises because the difference in electronegativity.

Molecules with lone pairs on the central atom Let us consider the molecules in which there are lone pairs on the central atoms. Note: Molecules which have lone pairs are usually polar.

Example: Ammonia ( effect of lone pairs ) 0


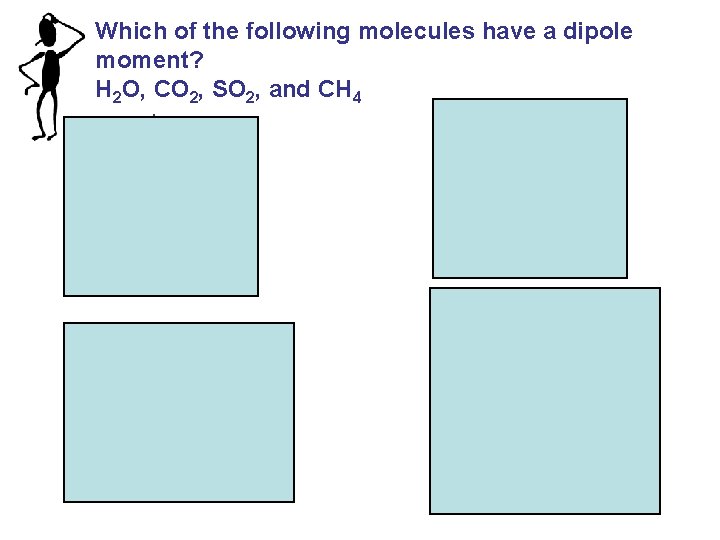
Which of the following molecules have a dipole moment? H 2 O, CO 2, SO 2, and CH 4 S O O H H dipole moment polar molecule O C O no dipole moment nonpolar molecule O dipole moment polar molecule H H C H H no dipole moment nonpolar molecule

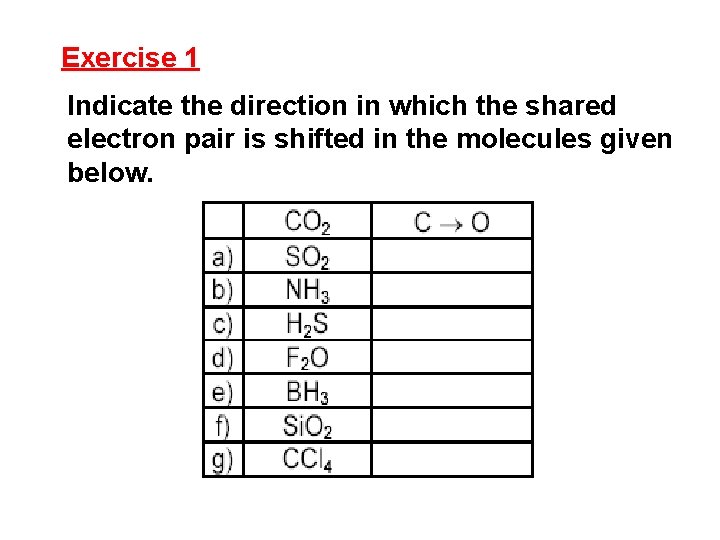
Exercise 1 Indicate the direction in which the shared electron pair is shifted in the molecules given below.

Answer:

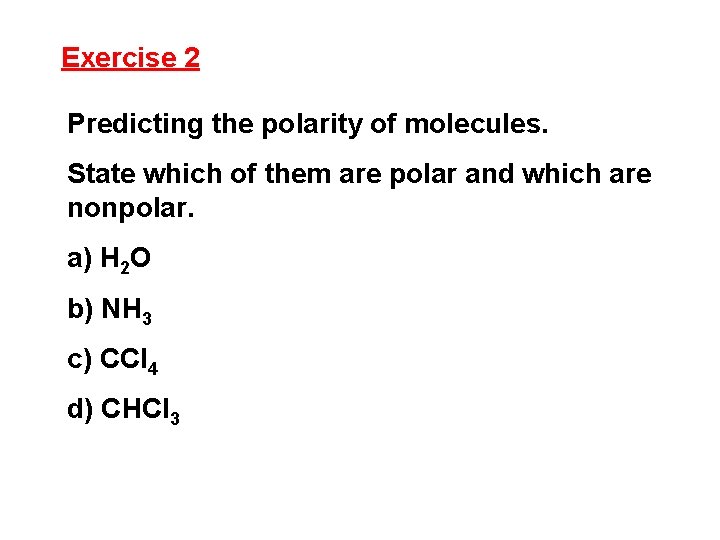
Exercise 2 Predicting the polarity of molecules. State which of them are polar and which are nonpolar. a) H 2 O b) NH 3 c) CCl 4 d) CHCl 3

Answer:
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Rich man poor man bible
Rich man poor man bible Rich tudor houses facts
Rich tudor houses facts Life in the roman empire
Life in the roman empire Data rich information poor
Data rich information poor Rich dad poor dad expense sheet
Rich dad poor dad expense sheet Explain why some countries are rich while others are poor
Explain why some countries are rich while others are poor Rich and poor tudors
Rich and poor tudors What did the tudors eat
What did the tudors eat Poor vs rich
Poor vs rich Relational mindset
Relational mindset Rich tudor food
Rich tudor food Rich and poor peter singer
Rich and poor peter singer Tudor rich and poor
Tudor rich and poor Why are some countries rich and others poor
Why are some countries rich and others poor Is h2o a polar molecule
Is h2o a polar molecule Why is water polar
Why is water polar Electronegativity of n-h
Electronegativity of n-h No2-1 molecular geometry
No2-1 molecular geometry Langevin-debye equation
Langevin-debye equation Non polar molecules that include fats oils and cholesterol
Non polar molecules that include fats oils and cholesterol