Polar Covalent Bonds Acids and Bases Bond Moments
Polar Covalent Bonds; Acids and Bases Bond Moments and Dipole Moments Formal Charge Resonance Bronsted-Lowry Acid/Base Lewis Acid/Base

Bonding Pattens for C, N, and O

Quick Review

Common Cationic, Neutral and Anionic Forms

Pauling Electronegativity Scale

Electronegativity Trends Ability to Attract the Electrons in a Covalent Bond

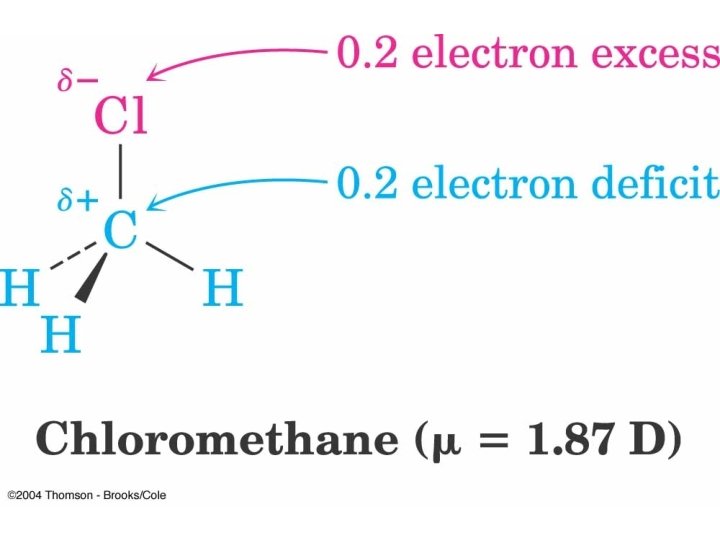
Electrostatic Potential Map Cholormethane

Opposite polarity in CH 3 Li

Methanol

Dipole Moment (m) is sum of the Bond Moments

Nonpolar Compounds Bond Moments Cancel Out

NCl 3 and BCl 3

Nitromethane has 2 Formal Charges

Both Resonance Structures Contribute to the Actual Structure

Dipole Moment reflects Both Resonance Structures
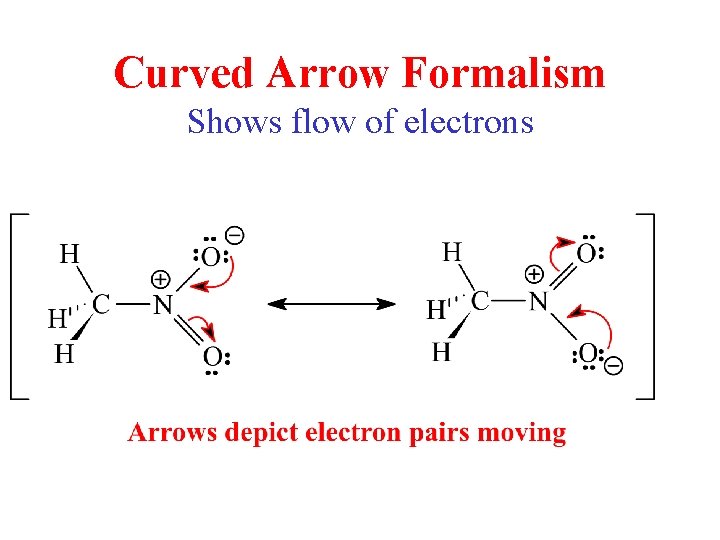
Curved Arrow Formalism Shows flow of electrons

Resonance Rules • Cannot break single (sigma) bonds • Only electrons move, not atoms 3 possibilities: – Lone pair of e- to adjacent bond position • Forms p bond - p bond to adjacent atom - p bond to adjacent bond position
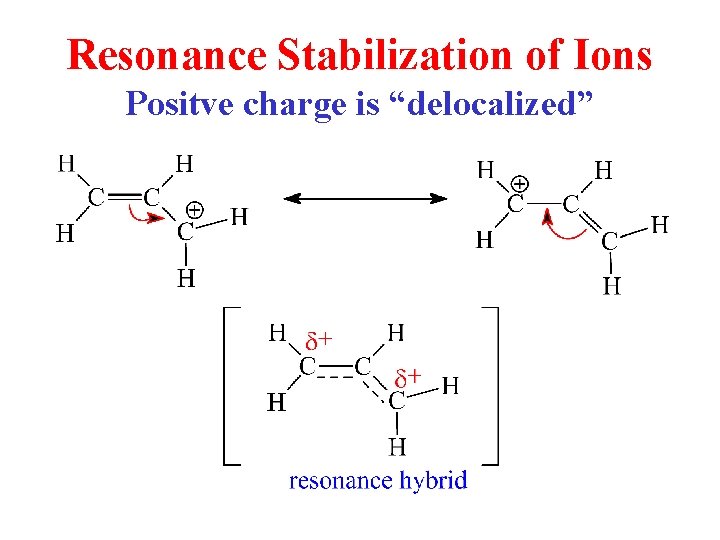
Resonance Stabilization of Ions Positve charge is “delocalized”

Definitions of Acids/Bases
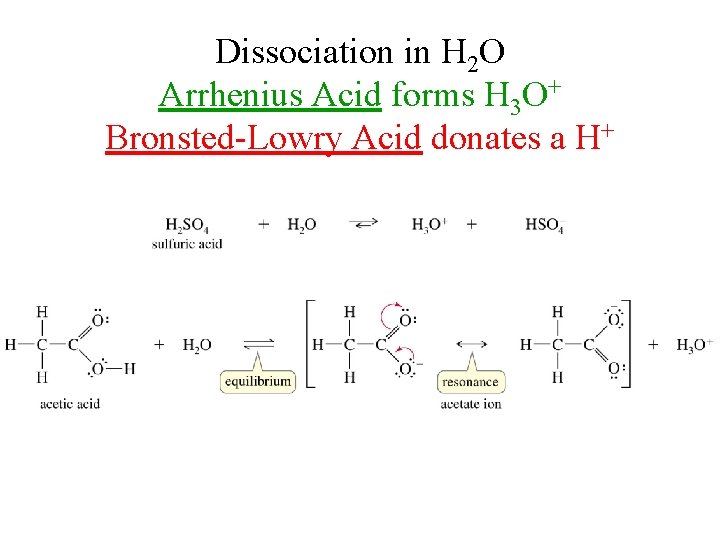
Dissociation in H 2 O Arrhenius Acid forms H 3 O+ Bronsted-Lowry Acid donates a H+

Reaction Described with Arrows

Lewis Acids and Bases
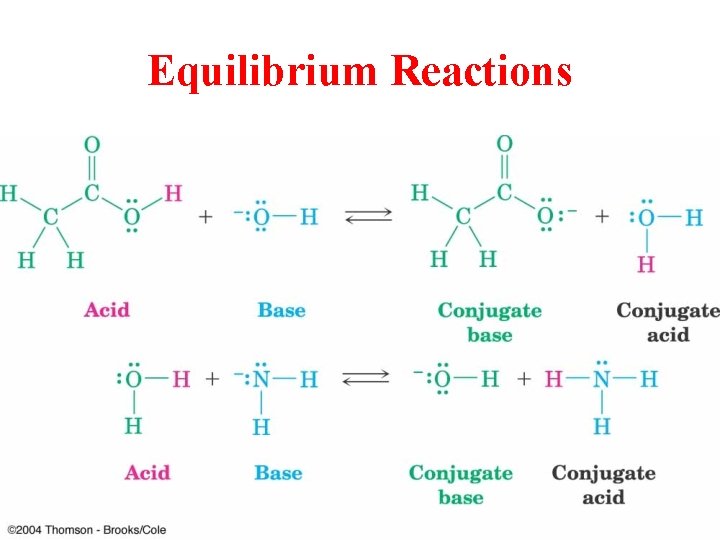
Equilibrium Reactions

Measuring Acid Strength Ka

Acid Strength defined by p. Ka



Resonance in Acetate Anion

Resonance Stabilization
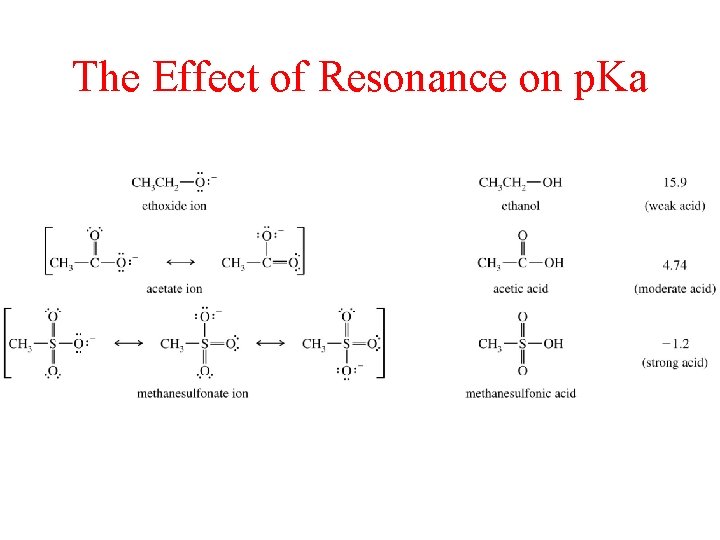
The Effect of Resonance on p. Ka
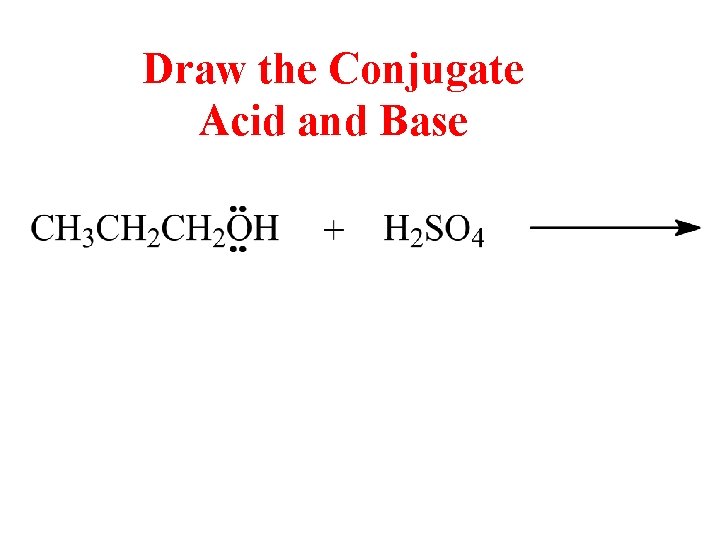
Draw the Conjugate Acid and Base
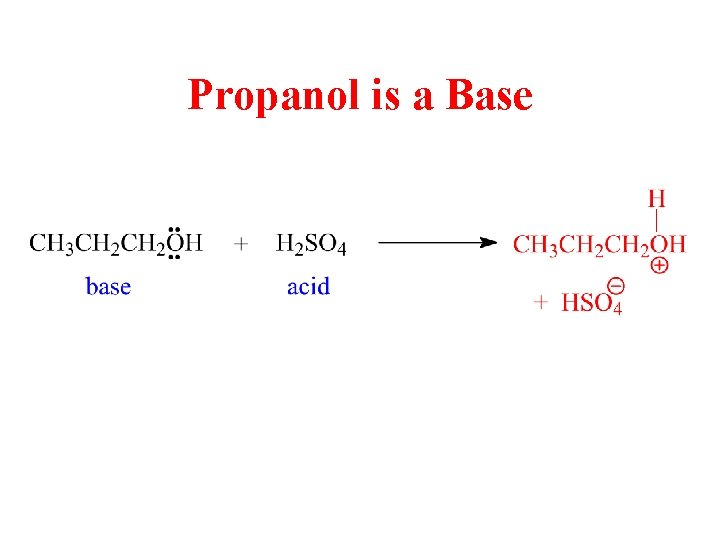
Propanol is a Base

Draw the Conjugate Acid and Base
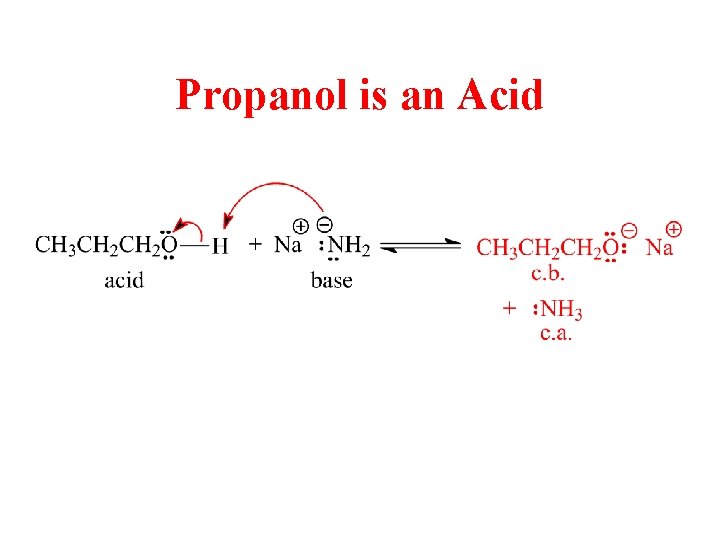
Propanol is an Acid
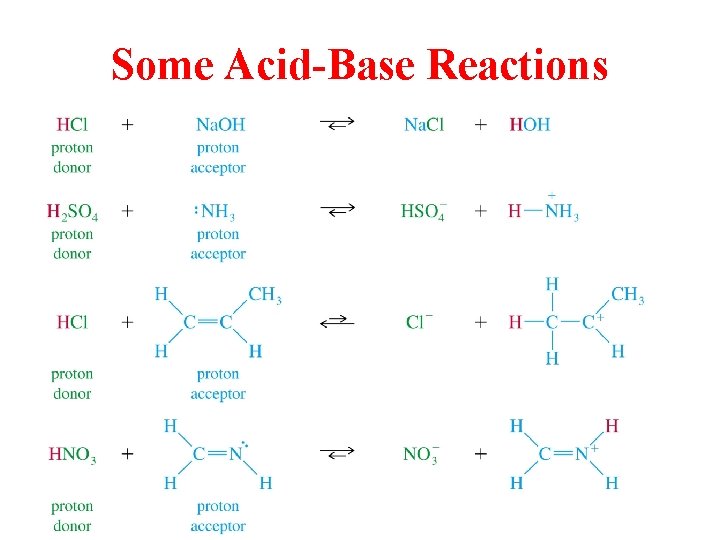
Some Acid-Base Reactions
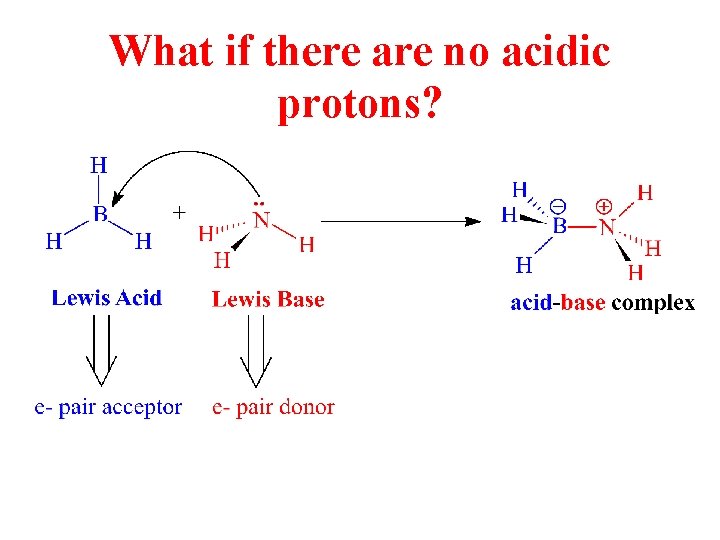
What if there are no acidic protons?
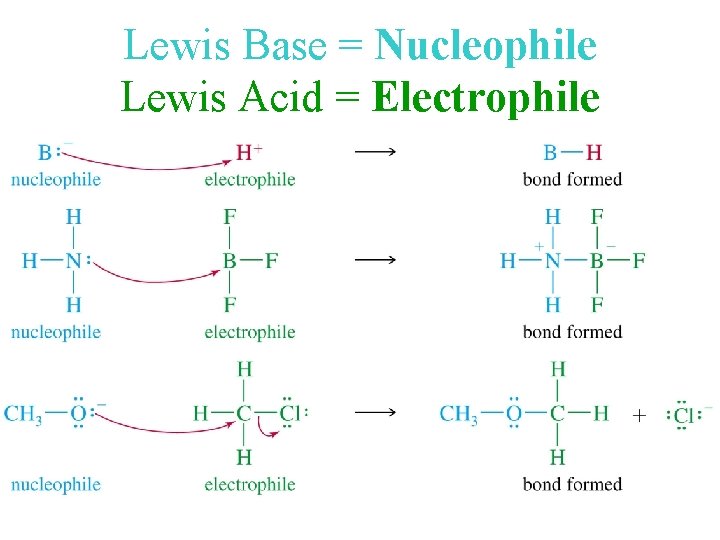
Lewis Base = Nucleophile Lewis Acid = Electrophile
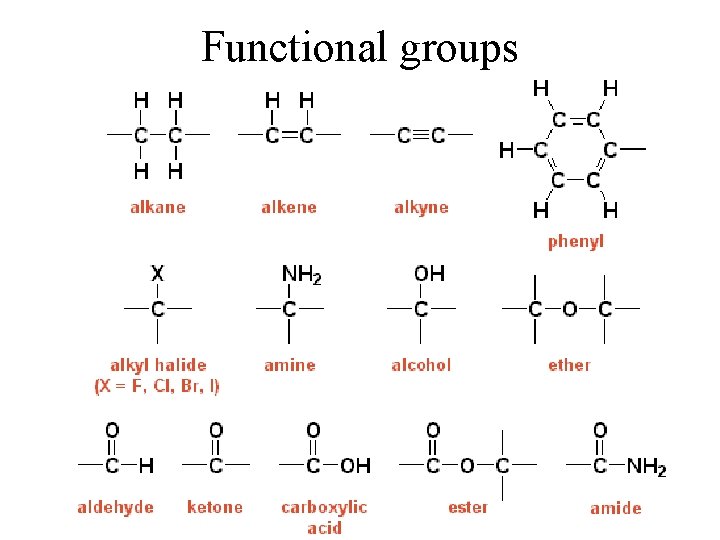
Functional groups


Functional groups determine chemistry

What will most likely happen if I add Br 2?

What will most likely happen if I add HCl?
- Slides: 43