Polar Bonds and Molecules Chapter 8 4 Learning
Polar Bonds and Molecules Chapter 8. 4

Learning Objectives • Be able to use electronegativity to identify polar vs. non-polar covalent bond • Draw correct dipoles on a covalent bond • Draw correct net dipole on a molecular compound
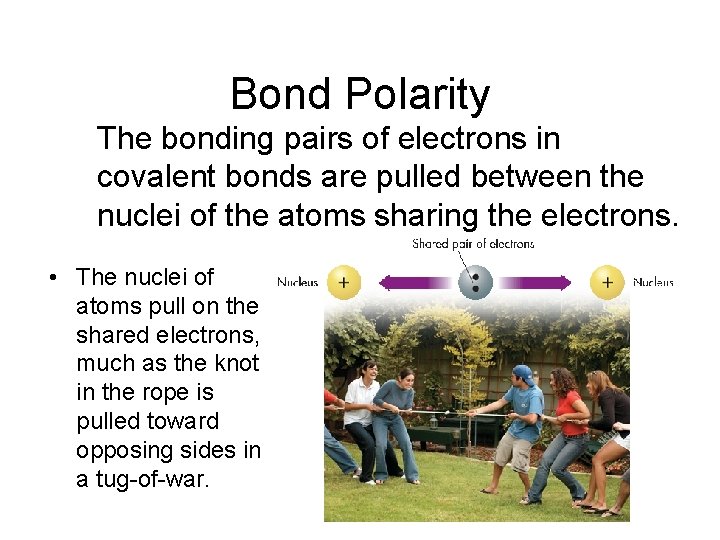
Bond Polarity The bonding pairs of electrons in covalent bonds are pulled between the nuclei of the atoms sharing the electrons. • The nuclei of atoms pull on the shared electrons, much as the knot in the rope is pulled toward opposing sides in a tug-of-war.
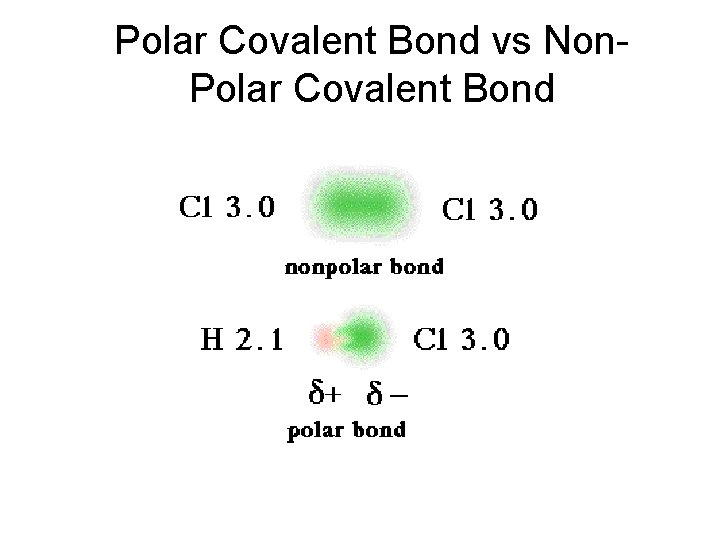
Bond Polarity The bonding pairs of electrons in covalent bonds are pulled between the nuclei of the atoms sharing the electrons. – When the atoms in the bond pull equally (as occurs when identical atoms are bonded), the bonding electrons are shared equally, and each bond formed is a nonpolar covalent bond.
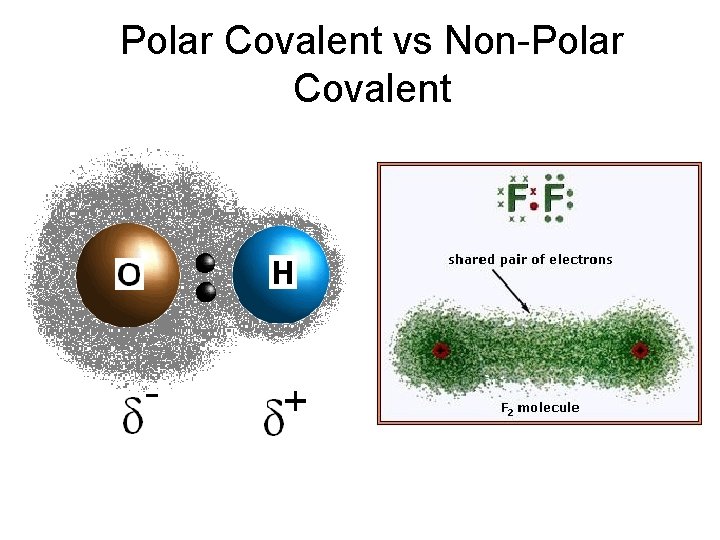
Bond Polarity A polar covalent bond, known also as a polar bond, is a covalent bond between atoms in which the electrons are shared unequally.
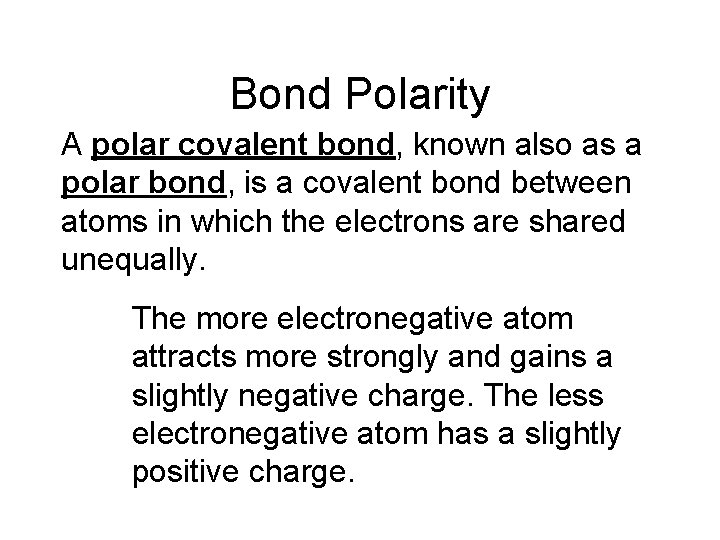
Bond Polarity A polar covalent bond, known also as a polar bond, is a covalent bond between atoms in which the electrons are shared unequally. The more electronegative atom attracts more strongly and gains a slightly negative charge. The less electronegative atom has a slightly positive charge.

Bond Polarity The higher the electronegativity value, the greater the ability of an atom to attract electrons to itself.

• Read pages 181 • Define electronegativity. • Copy the element symbol, atomic number and electronegativity values (pg 181) of the first 20 elements onto a periodic table.

Bond Polarity Describing Polar Covalent Bonds Hydrogen has an electronegativity of 2. 1, and chlorine has an electronegativity of 3. 0. • These values are significantly different, so the covalent bond in hydrogen chloride is polar. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
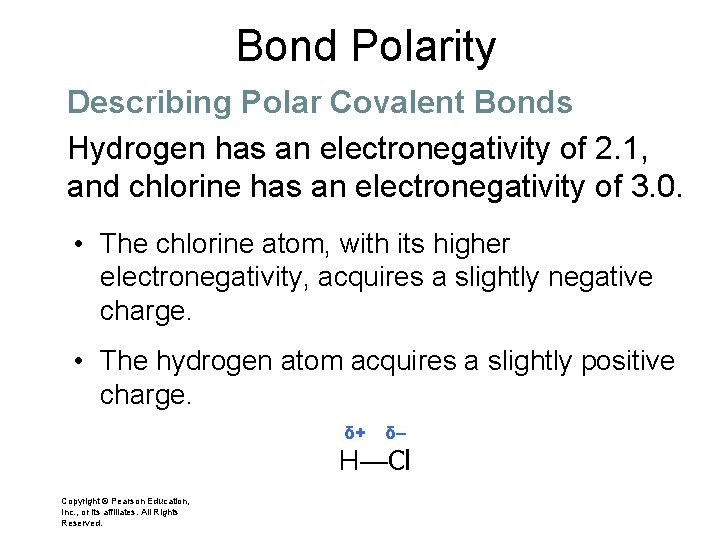
Bond Polarity Describing Polar Covalent Bonds Hydrogen has an electronegativity of 2. 1, and chlorine has an electronegativity of 3. 0. • The chlorine atom, with its higher electronegativity, acquires a slightly negative charge. • The hydrogen atom acquires a slightly positive charge. δ+ δ– H—Cl Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Bond Polarity Describing Polar Covalent Bonds The lowercase Greek letter delta (δ) denotes that atoms in the covalent bond acquire only partial charges, less than 1+ or 1–. δ+ δ– H—Cl • The minus sign shows that chlorine has a slightly negative charge. • The plus sign shows that hydrogen has acquired a slightly positive charge.
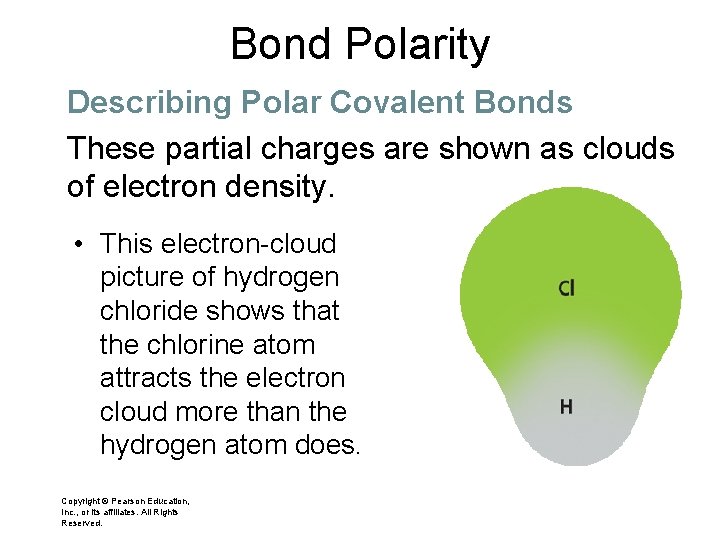
Bond Polarity Describing Polar Covalent Bonds These partial charges are shown as clouds of electron density. • This electron-cloud picture of hydrogen chloride shows that the chlorine atom attracts the electron cloud more than the hydrogen atom does. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
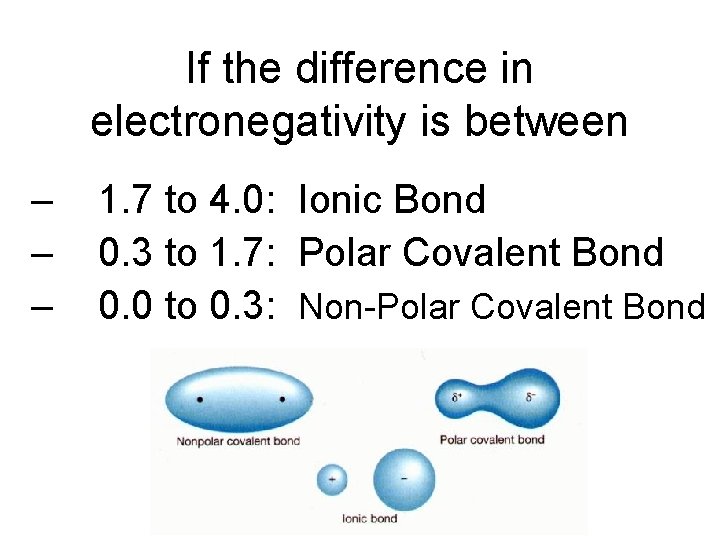
If the difference in electronegativity is between – – – 1. 7 to 4. 0: Ionic Bond 0. 3 to 1. 7: Polar Covalent Bond 0. 0 to 0. 3: Non-Polar Covalent Bond

Electronegativity Scale

Using
Polar Covalent Bond vs Non. Polar Covalent Bond
Polar Covalent vs Non-Polar Covalent
Drawing Bond Dipoles: BF 3
Drawing Bond Dipoles: CH 3 OH
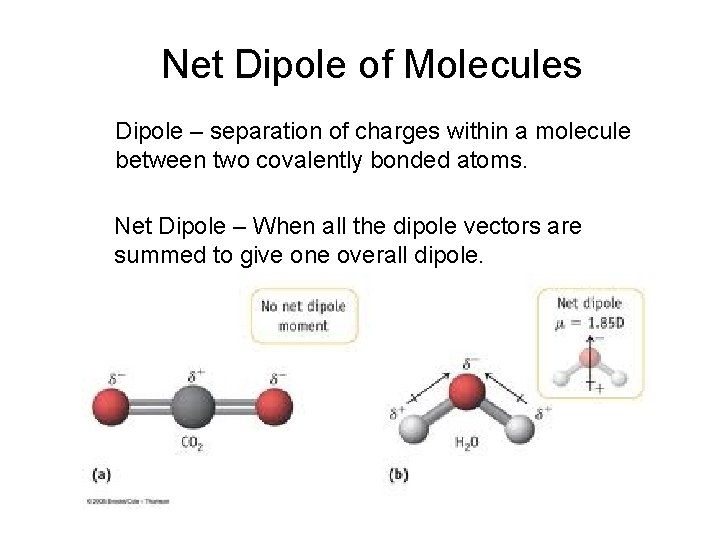
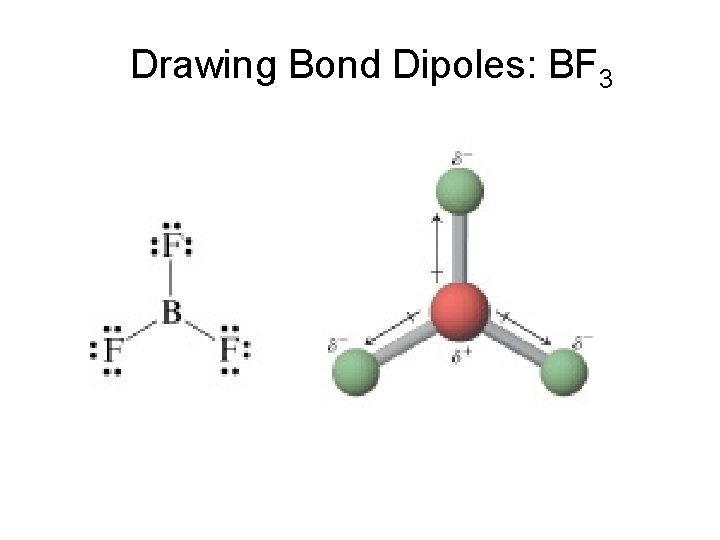
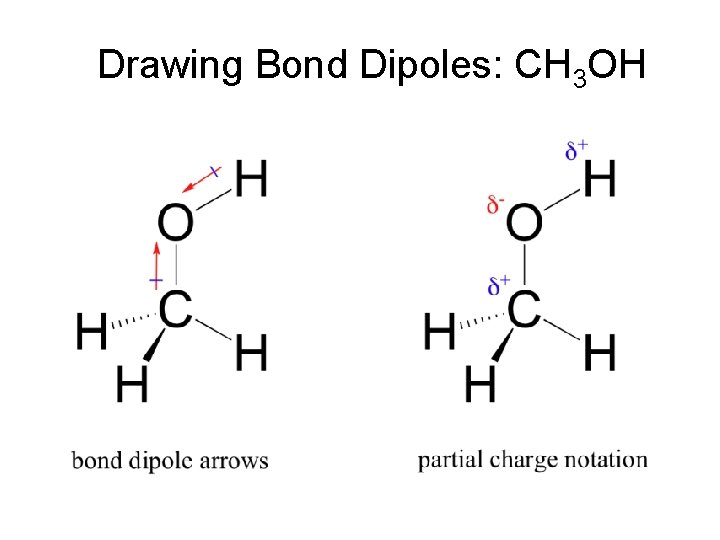
Net Dipole of Molecules Dipole – separation of charges within a molecule between two covalently bonded atoms. Net Dipole – When all the dipole vectors are summed to give one overall dipole.
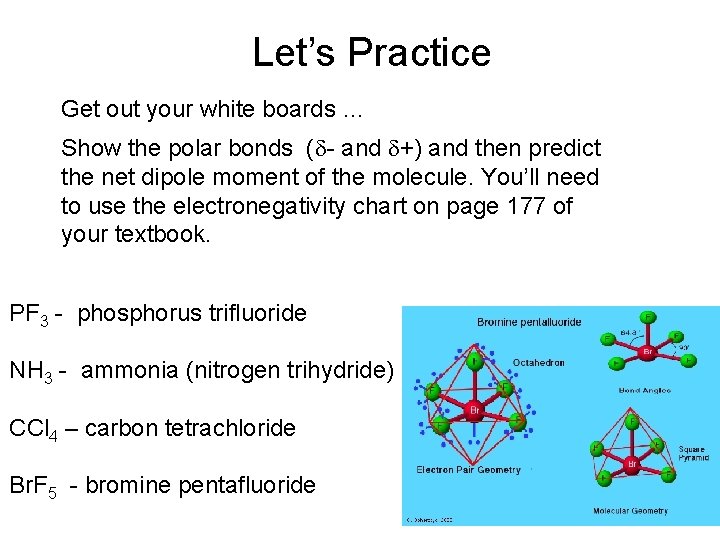
Let’s Practice Get out your white boards … Show the polar bonds (d- and d+) and then predict the net dipole moment of the molecule. You’ll need to use the electronegativity chart on page 177 of your textbook. PF 3 - phosphorus trifluoride NH 3 - ammonia (nitrogen trihydride) CCl 4 – carbon tetrachloride Br. F 5 - bromine pentafluoride
- Slides: 21