Point of Care Testing POCT Prepared By Rushika

Point of Care Testing (POCT) Prepared By • • Rushika Savaliya Kinjal Thummar Radhika Trapasiya Bhumika Reshamwala Kerul Pambhar Kinjal Radadiya Sharmila Das

• Definition • Instrument Type, Name & Test • Principle of the instrument • Advantage • Disadvantage • Calibration of the POCT instrument • Quality control • Procedure , Material , Frequency , Level, Evaluation • Documentation & Process require for accreditation of POCT instrument

DEFINATION • Testing conducted outside a lab • In a hospital /in a clinic • Performance of tests • “bed side” • “near the site of care. ”
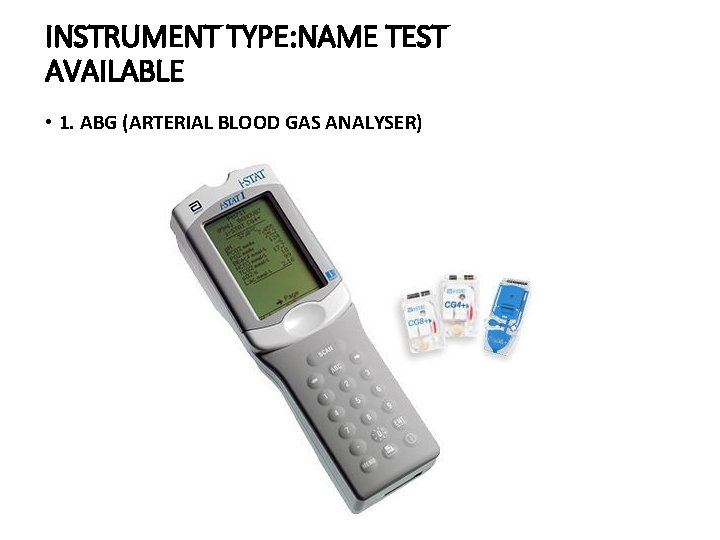
INSTRUMENT TYPE: NAME TEST AVAILABLE • 1. ABG (ARTERIAL BLOOD GAS ANALYSER)
ABG – POCT principle • Mainly “Potentiometric electrodes” • p. H & Naᶧ ‐‐‐ Special glass membrane • Kᶧ & Caᶧᶧ ‐‐‐ Special membrane with neutral carriers • Cl‾ ‐‐‐ Ion exchange used in Cl‐ membrane • p. CO 2 ‐‐‐ Same membrane like p. H , with inter membrane buffer. • p. O 2 ‐‐‐ Clark electrode to measure current

Principle of POCT for Glucose & Lactate • Glucose and Lactate glucose oxidase Glucose ‐‐‐‐‐‐‐‐‐‐‐‐ Glucono lactone + H 2 O 2 lactate oxidase Lactate ‐‐‐‐‐‐‐‐‐‐‐‐‐‐ pyruvate +H 2 O 2 determined by Amperometrically With Manganese dioxide/Carbon electrode at 350 m. V.
(2) Automated Urinary analysis : q. Consists of an optical system q. Incandescent lamp & photo‐diode pack containing four filter • Blue (400‐ 510 nm) • Green (510‐ 586 nm) • Red (586‐ 660 nm) • Infrared (825‐ 855 nm) q. Lamp illuminates a light of fix wave on “read area” q. That reflects light back into the photodiode pack. q. Photodiode convert it into electrical current ‐ potential.
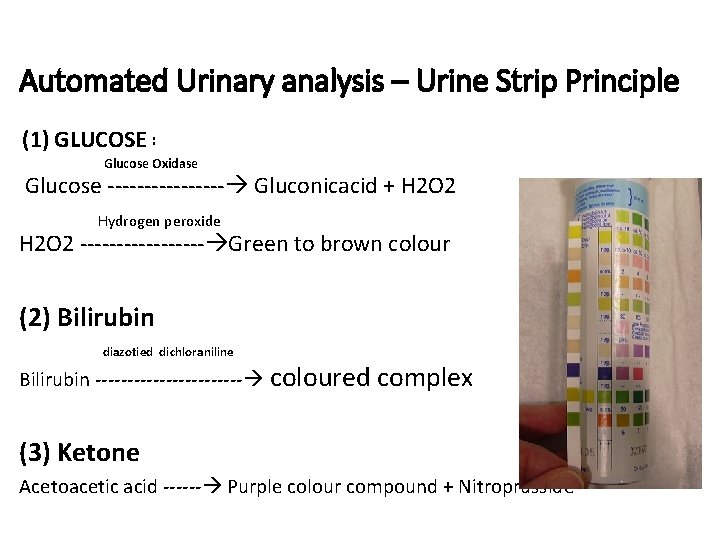
Automated Urinary analysis – Urine Strip Principle (1) GLUCOSE : Glucose Oxidase Glucose ‐‐‐‐‐‐‐‐ Gluconicacid + H 2 O 2 Hydrogen peroxide H 2 O 2 ‐‐‐‐‐‐‐‐‐ Green to brown colour (2) Bilirubin diazotied dichloraniline Bilirubin ‐‐‐‐‐‐‐‐‐‐‐‐ coloured complex (3) Ketone Acetoacetic acid ‐‐‐‐‐‐ Purple colour compound + Nitroprusside
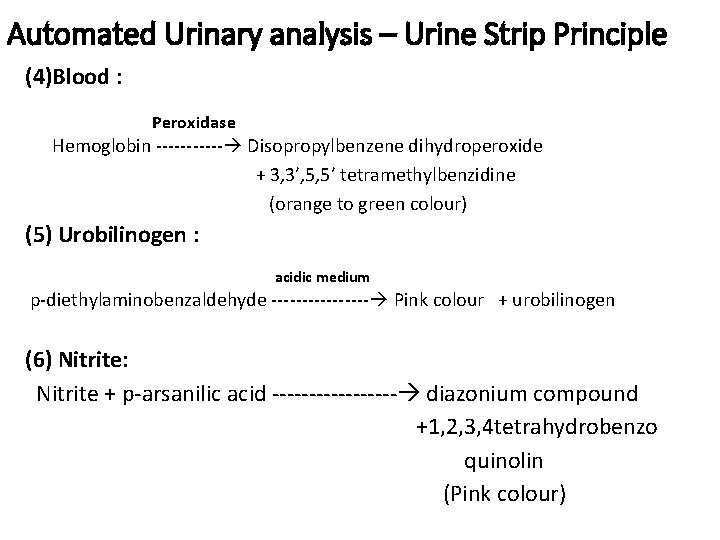
Automated Urinary analysis – Urine Strip Principle (4)Blood : Peroxidase Hemoglobin ‐‐‐‐‐‐ Disopropylbenzene dihydroperoxide + 3, 3’, 5, 5’ tetramethylbenzidine (orange to green colour) (5) Urobilinogen : acidic medium p‐diethylaminobenzaldehyde ‐‐‐‐‐‐‐‐ Pink colour + urobilinogen (6) Nitrite: Nitrite + p‐arsanilic acid ‐‐‐‐‐‐‐‐‐ diazonium compound +1, 2, 3, 4 tetrahydrobenzo quinolin (Pink colour)

(3) GLUCOMETER - PRINCIPLE NAD‐ Glucose dehydrogenase Glucose + NAD‐‐‐‐‐‐‐‐‐ Gluconolactone + NADH + Phenanthroline Quanone Electrical potential is generated in strip Which reflect glucose concentration in mg/ml.
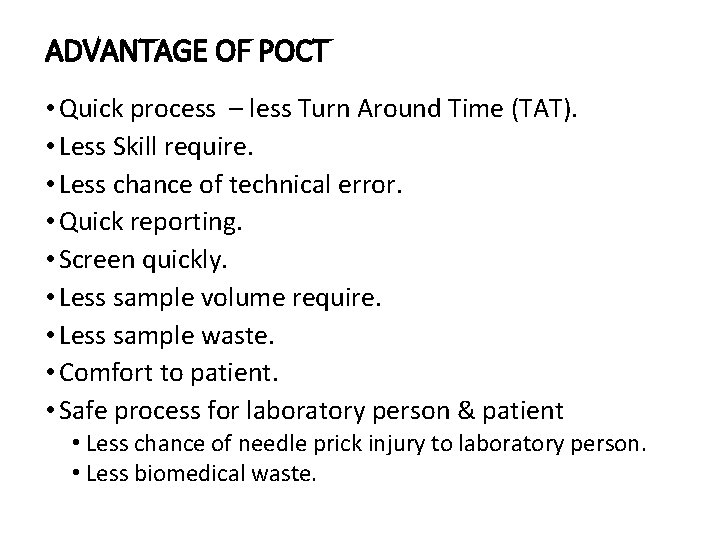
ADVANTAGE OF POCT • Quick process – less Turn Around Time (TAT). • Less Skill require. • Less chance of technical error. • Quick reporting. • Screen quickly. • Less sample volume require. • Less sample waste. • Comfort to patient. • Safe process for laboratory person & patient • Less chance of needle prick injury to laboratory person. • Less biomedical waste.

DISADVANTAGE • Higher cost. • Lack of comparability with quality control. • Difficult to retrieve previous data. • Lower accuracy and precision e. g. Glucometer. • Difficulty in assuring quality- E. g. Urine strip.

CALIBRATION OF POCT INSTRUMENT When To Do • Calibrated before it leaves the factory. • After installation • Calibration of Strips before you begin to read test strips. • Must be recalibrated instrument every yearly • According to manufacturer guideline.
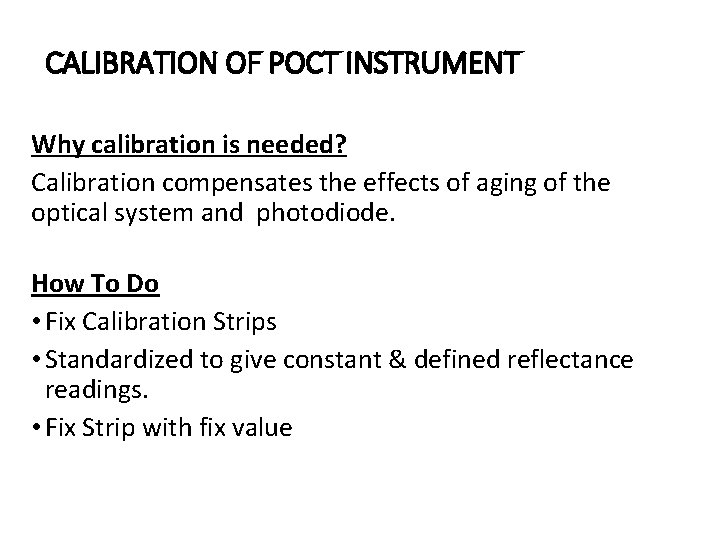
CALIBRATION OF POCT INSTRUMENT Why calibration is needed? Calibration compensates the effects of aging of the optical system and photodiode. How To Do • Fix Calibration Strips • Standardized to give constant & defined reflectance readings. • Fix Strip with fix value

QUALITY CONTROL Need of Quality Control in POCT • When designing QC strategy following to be considered • High sensitive test. • Frequency of test. • Manufacturer guideline
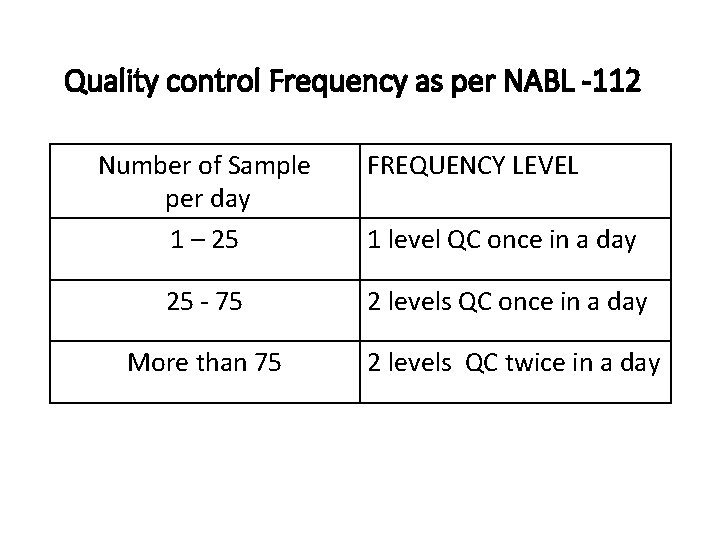
Quality control Frequency as per NABL -112 Number of Sample per day 1 – 25 FREQUENCY LEVEL 1 level QC once in a day 25 ‐ 75 2 levels QC once in a day More than 75 2 levels QC twice in a day
POCT devices are divided into 3 categories: 1. High Throughput Analyzer • Full sized instruments used as a point of care • Blood gas analyzer. 2. Cartridge Based Instruments • Hb. A 1 c analyzer. 3. Strip based instruments • Electrochemical strip based glucose meters. 4. Manually read tests • Urine dip‐stick test.

1) High throughput analyzer • Those analyzer are seen within the laboratory. • But instrument like Arterial Blood Gas Analyzer require in ICCU as well as in operation theater per sensitivity of the test • Quality Control Standard • NABL – 112 (Specific Criteria for Medical Laboratory) • Multilevel third party controls • Depending on the test and number of samples processed • For rare parameter – less sample frequency • At least one level one time before the process
2) Cartridge Based Instruments • Cartridge based instrument contains all the necessary ingredients for analysis of patient samples. • The electronic reader component is responsible for converting the result from cartridge component into numerical value for analysis. • Quality control guideline • Similar to High throughput analyzer • Some cartridge devices have their own inbuilt QC self‐ check function • Ex: Hb. A 1 c analyzer & Mini Vidas Analyzer.


3) Strip Based Instruments • Quality control guideline • Similar to High throughput analyzer • Unlike cartridge based instruments, strip based instrument has no self check quality control feature. • So QC processes should be done more strictly • Example : electrochemical strip based glucose meters.
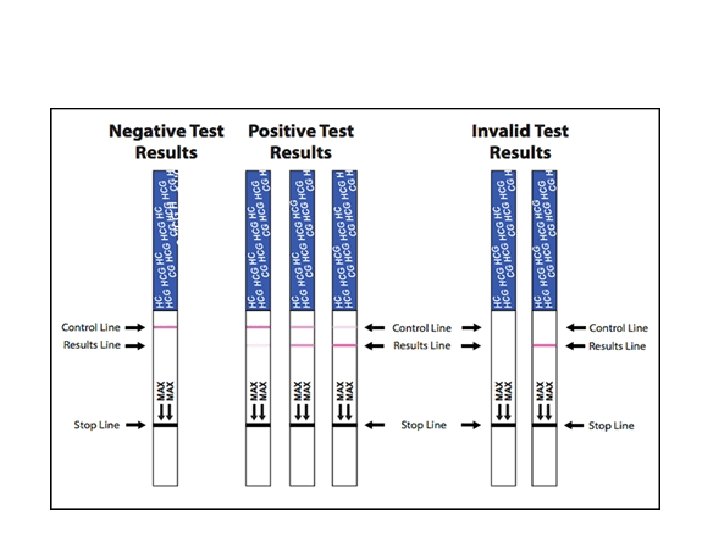

4) Manually Read Test: • Strip may have in‐built control slot to check functioning of the strip. • Most of the strip give qualitative result • Negative & Positive • Essential to run Positive & Negative QC • As Internal Quality Control as well as External Quality Assurance • Example : Urine dipstick test • Glucose , Protein , Ketone body • Beta HCG – Pregnancy test

Choosing IQC material and an EQA scheme for POCT devices 1. Choosing IQC material for POCT devices • A matrix similar to the patient sample • Clinically relevant concentrations • QC must have defined accurate value • Multi Level QC • Third party QC
2. Choosing an EQAS scheme for POCT devices • At least once in month participation in EQAS • Frequency is depend on • Number of sample • Sensitivity of test • Quality material suitable for use with POCT devices. • EQAS programme has participation of • multiple instrument participation with different principle • More accurate instrument • Non‐POCT instrument
Accreditation of POCT instrument • NABL 112 & ISO 15189: 2012 – Criteria require • Documentation & Implementation of IQC & EQAS participation • Procedure of Quality Control • Data of Quality control • Evaluation of Quality Control • L‐J chart draw • Quantitative value • Qualitative value – Reflextion value – O. D. • Root cause analysis in case of IQC & EQAS outlier
- Slides: 26