Please mute yourselves on phone or computer Thank





















- Slides: 24

Please mute yourselves, on phone or computer. Thank you.

Performance Improvement Project (PIP) Clinic: Indicators and Outcomes June 19, 2015 Amy Mc. Curry Schwartz, Esq. , MHSA EQRO Consultant Behavioral Health Concepts, Inc. amy. mccurry@bhceqro. com

Performance Improvement Projects The purpose of PIPs To assess and improve processes, and thereby outcomes, of care. 42 CFR 438. 240(d) defines PIPs as having a “focus on clinical and nonclinical areas. ”

Performance Improvement Projects Clinical PIPs Might Target Prevention and care of acute and chronic conditions High-volume services High-risk procedures Special health care needs

Clinical PIPs While enrollee satisfaction is an important outcome of care in clinical areas, improvement in satisfaction should not be the only measured outcome of a clinical project. Some improvement in health or functional status should be addressed. CMS Protocol 3: Validating Performance Improvement Projects (PIPs), September 2012

Performance Improvement Projects Non-Clinical PIPs Might Target Coordination of Care Appeals, Grievances Process Access or Authorization Member Services

Non-Clinical PIPs For projects in non-clinical areas, the use of health or functional status measures is preferred…when addressing access or availability status. Enrollee satisfaction alone may be sufficient for some non-clinical projects. CMS Protocol 3: Validating Performance Improvement Projects (PIPs), September 2012

Questions

Select the Study Indicator(s) A study indicator is: A measurable characteristic, quality, trait, or attribute of a particular individual, object, or situation being studied.

Study Indicators may be quantitative or qualitative and continuous or discrete. Discrete or categorical indicators have a limited number of possible categories An individual has/has not received a flu shot in the last 12 months.

Study Indicators Continuous indicators have unlimited possible values within the limits of the indicator range Age Blood Pressure Temperature Data collected on a continuous indicator such as blood pressure can be used for a discrete indicator (e. g. a consumer’s blood pressure is/is not below a specific level)

Study Indicators Criteria Each PIP should have one or more measured indicators to track performance and improvement over a specific period of time. All measured indicators should be: Objective; and Clearly defined; and Based on current clinical knowledge or health services research; and Enrollee outcomes (e. g. health or functional status, enrollee satisfaction); or a valid indicator of these outcomes.

Study Indicators The number and complexity of indicators (measures) may vary depending on: The Study Question(s); The complexity of existing practice guidelines for a clinical condition; and Availability of data and resources to gather data

Study Indicators Potential Sources of Supporting Information o o o Clinical and non-clinical practice guidelines o http: //www. psychiatry. org/practice/clinical-practiceguidelines o http: //www. cdc. gov/chronicdisease/resources/guideline s. htm Administrative Data o Claims data o Enrollment files Medical Records

Developing Appropriate Indicators Examples of measures currently existing within the public health community or managed care industry include: NCQA Healthcare Effectiveness Data Information Set (HEDIS) www. ncqa. org Measures developed by CMS and AHRQ (Pediatric or Adult Core Measures) www. ahrq. gov

Questions

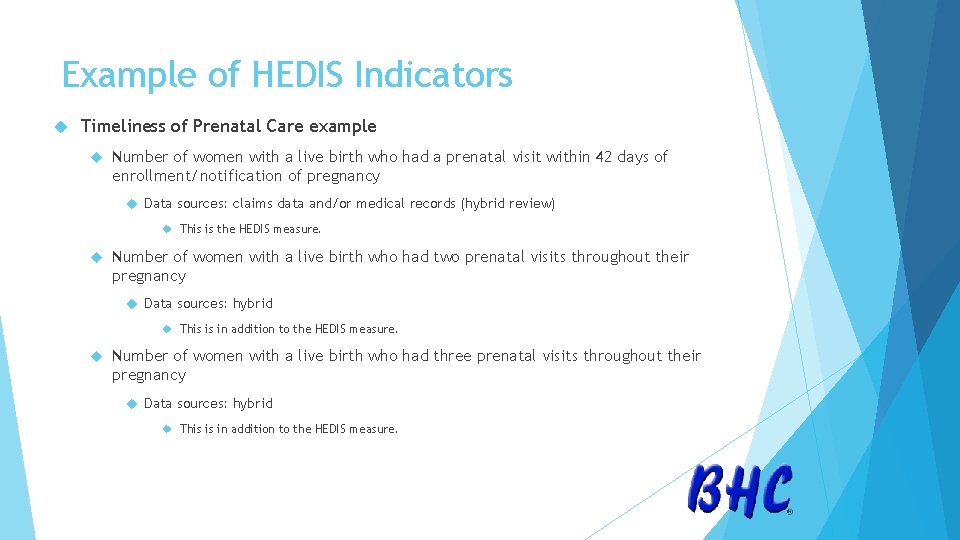
Example of HEDIS Indicators Timeliness of Prenatal Care example Number of women with a live birth who had a prenatal visit within 42 days of enrollment/notification of pregnancy Data sources: claims data and/or medical records (hybrid review) This is the HEDIS measure. Number of women with a live birth who had two prenatal visits throughout their pregnancy Data sources: hybrid This is in addition to the HEDIS measure. Number of women with a live birth who had three prenatal visits throughout their pregnancy Data sources: hybrid This is in addition to the HEDIS measure.

Developing Study Indicators The MHP may also develop measures based on current clinical practice guidelines or health services research. When developing your own measures, the MHP must document the basis for adoption. Consider the following questions: A. Did the study use objective, clearly defined, measureable, timespecific indicators? B. Do the measures capture changes in health status, functional status, or enrollee satisfaction?

Developing Study Indicators C. Do the measures have any of the following key characteristics: o Related to identified health care guidelines relevant to the study question? o An important aspect of care or operations that made a difference to the MHP’s consumers o Data available through administrative, medical records or another readily accessible source o Limitations on the ability to collect the data skew the results o Require explicit or implicit criteria o If relevant, a strategy to ensure inter-rater reliability

Example of Study Indicators If goal is: More participation in treatment Indicator – “On follow up visit, clinician reported the consumer was engaged in treatment and willing to work towards treatment goals. ” If goal is: More ability to recall what client was to accomplish before the next visit Indicator – “Consumer was able to accomplish 1 or more next steps in their treatment plan by their return visit”. If goal is: Consumer satisfaction Indicator – “Pre and post satisfaction testing”

Outcomes Changes in patient health, functional status, or satisfaction resulting from the PIP. Clinical PIPs Measures should include a change in health status or functional status or a process of care proxy. Standardized performance measures addressing outcomes may be limited because health outcomes are influenced by factors outside of the organization’s control, such as poverty, genetics, and environment. For these reasons, quality measures do not always need to be outcome measures.

Outcomes Process measures, while acceptable, must offer strong clinical evidence that the process being measured is meaningfully associated with outcomes This determination should be based on published guidelines, including citations from randomized clinical trials, case control studies, or cohort studies.

Outcomes The PIP should demonstrate a consensus among relevant practitioners with expertise in the defined area who attest to the importance of a given process. It is important that MHPs note within their PIP any potential external threats to validity which could affect the results of the outcome measures.

Questions