Platelet Function Testing Which one Should we Perform



- Slides: 20

Platelet Function Testing: Which one Should we Perform and how to Interpret the Data? Dominick J. Angiolillo, MD, Ph. D, FACC, FESC, FSCAI Director of Cardiovascular Research Assistant Professor of Medicine

DISCLOSURES Dominick J. Angiolillo, MD, Ph. D Within the past 12 months, the presenter or their spouse/partner have had a financial interest/arrangement or affiliation with the organization listed below. Honoraria/Lectures: Bristol Myers Squibb, Sanofi-Aventis, Eli Lilly Co, Daiichi Sankyo, Inc. , Advisory Board: Bristol Myers Squibb, Sanofi-Aventis, Eli Lilly Co, Daiichi Sankyo, Inc. , The Medicines Company, Portola Pharmaceuticals, Novartis, Arena Pharmaceuticals, Accumetrics, Medicure Research Grants: Glaxo. Smith. Kline, Otsuka, Accumetrics, Eli Lilly Co, Daiichi Sankyo, Inc. , The Medicines Company, Portola Pharmaceuticals, Schering-Plough, Astra Zeneca, Johnson & Johnson I intend to reference unlabeled/ unapproved uses of drugs or devices in my presentation. I intend to reference Ticagrelor, Elinogrel, Cangrelor, and TRA.

Antiplatelet Drug Resistance / Response Variability: An Emerging Clinical Problem

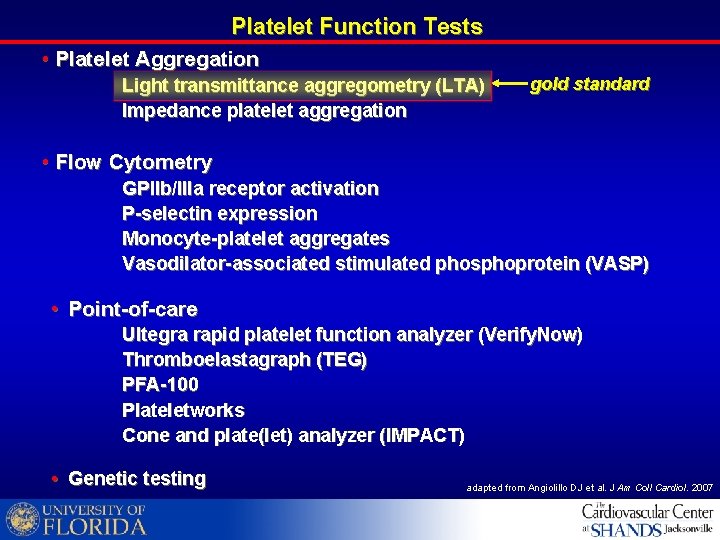
Platelet Function Tests • Platelet Aggregation Light transmittance aggregometry (LTA) Impedance platelet aggregation gold standard • Flow Cytometry GPIIb/IIIa receptor activation P-selectin expression Monocyte-platelet aggregates Vasodilator-associated stimulated phosphoprotein (VASP) • Point-of-care Ultegra rapid platelet function analyzer (Verify. Now) Thromboelastagraph (TEG) PFA-100 Plateletworks Cone and plate(let) analyzer (IMPACT) • Genetic testing adapted from Angiolillo DJ et al. J Am Coll Cardiol. 2007

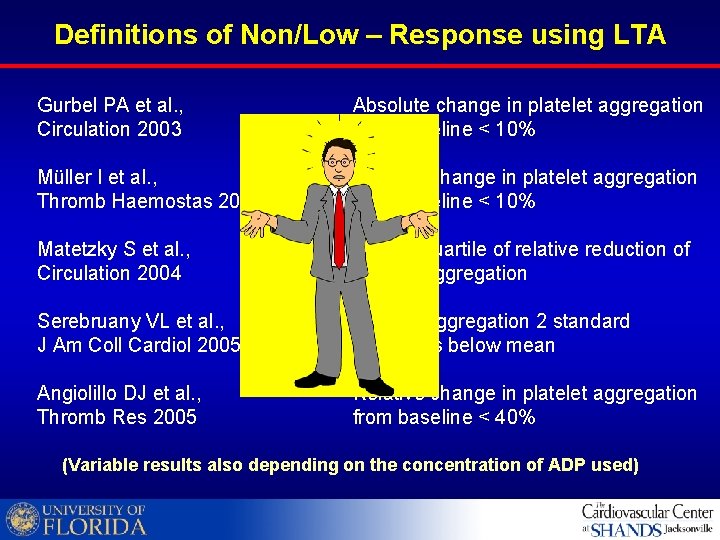
Definitions of Non/Low – Response using LTA Gurbel PA et al. , Circulation 2003 Absolute change in platelet aggregation from baseline < 10% Müller I et al. , Thromb Haemostas 2003 Relative change in platelet aggregation from baseline < 10% Matetzky S et al. , Circulation 2004 Lowest quartile of relative reduction of platelet aggregation Serebruany VL et al. , J Am Coll Cardiol 2005 Platelet aggregation 2 standard deviations below mean Angiolillo DJ et al. , Thromb Res 2005 Relative change in platelet aggregation from baseline < 40% (Variable results also depending on the concentration of ADP used)

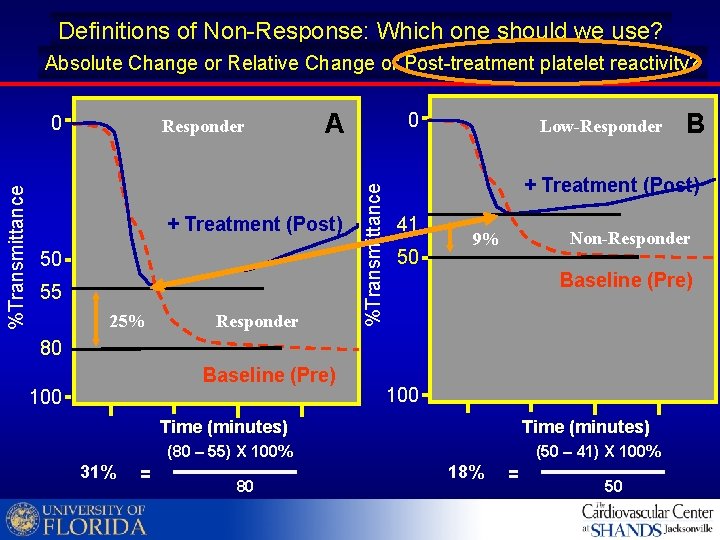
Definitions of Non-Response: Which one should we use? Absolute Change oror. Relative Change? Absolute Change or Relative Change Post-treatment platelet reactivity? Responder A + Treatment (Post) 50 55 25% Responder 0 %Transmittance 0 Low-Responder + Treatment (Post) 41 50 Non-Responder 9% Baseline (Pre) 80 Baseline (Pre) 100 Time (minutes) (80 – 55) X 100% 31% = 80 B (50 – 41) X 100% 18% = 50

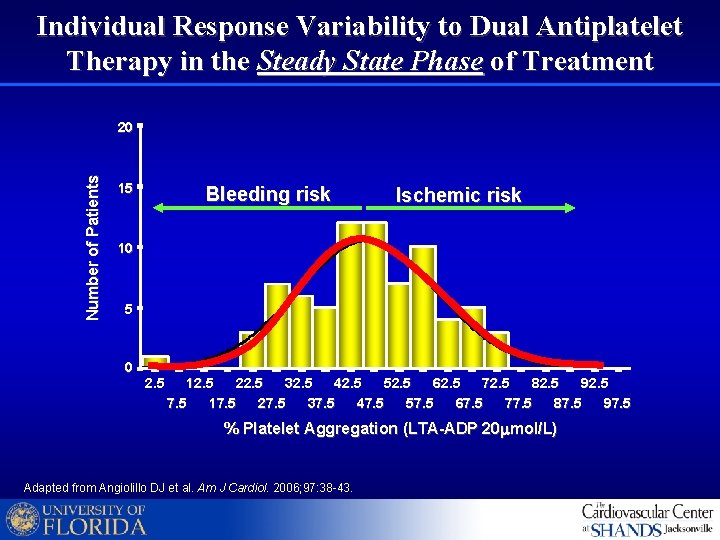
Individual Response Variability to Dual Antiplatelet Therapy in the Steady State Phase of Treatment Number of Patients 20 15 Bleeding risk Ischemic risk 10 5 0 2. 5 12. 5 22. 5 32. 5 42. 5 52. 5 62. 5 72. 5 82. 5 92. 5 7. 5 17. 5 27. 5 37. 5 47. 5 57. 5 67. 5 77. 5 87. 5 97. 5 % Platelet Aggregation (LTA-ADP 20 mmol/L) Adapted from Angiolillo DJ et al. Am J Cardiol. 2006; 97: 38 -43.

Optimizing Platelet Response in Suboptimal Responders • Modifying dosage of currently approved drugs (e. g. higher dose) • Adding other agents with antiplatelet properties (e. g. GPIIb/IIIa inhibitors; cilostazol) • Using alternative drugs (e. g. ticlopidine or novel antiplatelet agents)

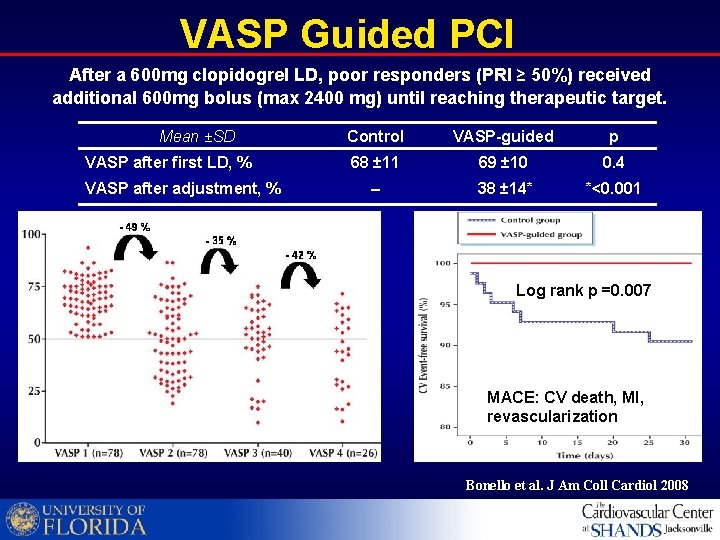
VASP Guided PCI After a 600 mg clopidogrel LD, poor responders (PRI ≥ 50%) received additional 600 mg bolus (max 2400 mg) until reaching therapeutic target. Mean ±SD VASP after first LD, % VASP after adjustment, % Control VASP-guided p 68 ± 11 69 ± 10 0. 4 38 ± 14* *<0. 001 Log rank p =0. 007 MACE: CV death, MI, revascularization Bonello et al. J Am Coll Cardiol 2008

VASP and LTA: limitations • • • Not-user friendly Time consuming Require experienced lab personnel Require expensive equipment Not universally available Overall, …. expensive

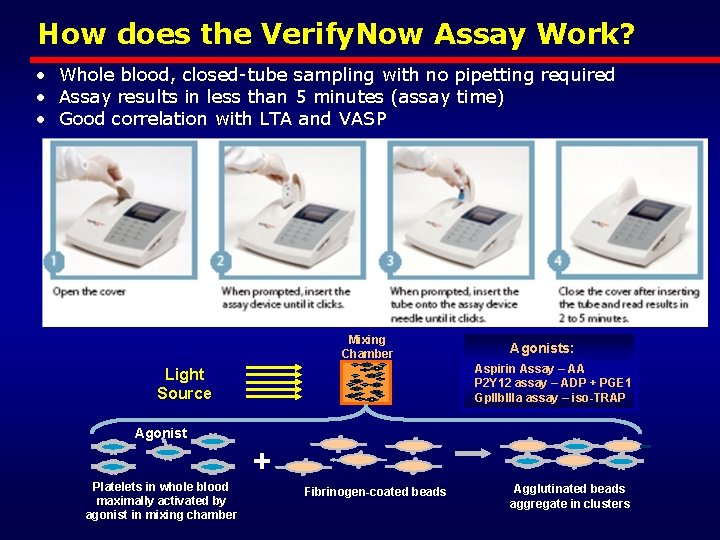
How does the Verify. Now Assay Work? • Whole blood, closed-tube sampling with no pipetting required • Assay results in less than 5 minutes (assay time) • Good correlation with LTA and VASP Mixing Chamber Agonists: Aspirin Assay – AA P 2 Y 12 assay – ADP + PGE 1 Gp. IIb. IIIa assay – iso-TRAP Light Source Agonist + Platelets in whole blood maximally activated by agonist in mixing chamber Fibrinogen-coated beads Agglutinated beads aggregate in clusters

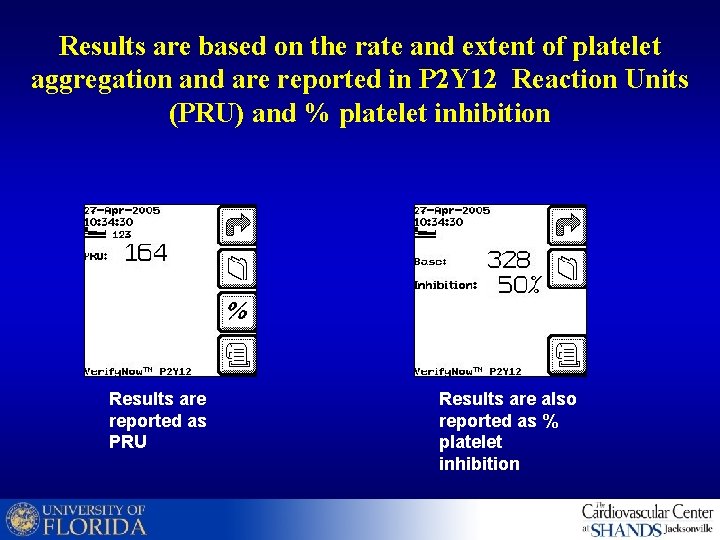
Results are based on the rate and extent of platelet aggregation and are reported in P 2 Y 12 Reaction Units (PRU) and % platelet inhibition Results are reported as PRU Results are also reported as % platelet inhibition

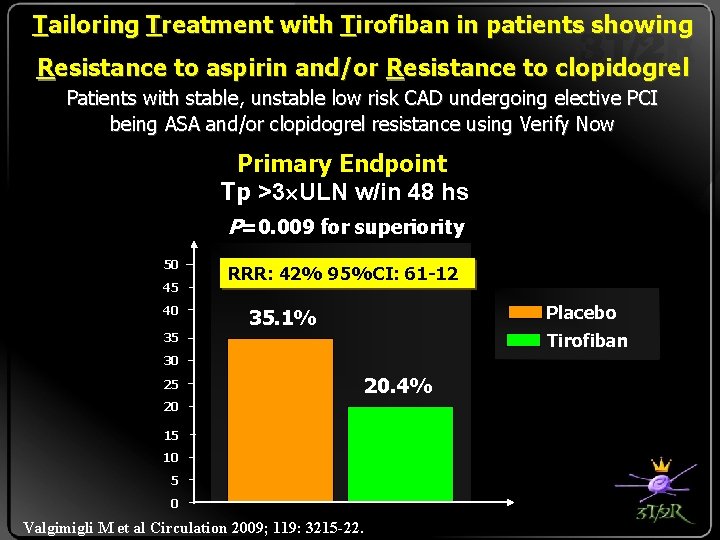
Tailoring Treatment with Tirofiban in patients showing Resistance to aspirin and/or Resistance to clopidogrel Patients with stable, unstable low risk CAD undergoing elective P CI being ASA and/or clopidogrel resistance using Verify Now Primary Endpoint Tp >3 ULN w/in 48 hs P=0. 009 for superiority 50 45 40 RRR: 42% 95%CI: 61 -12 Placebo 35. 1% Tirofiban 35 30 25 20 15 10 5 0 Valgimigli M et al Circulation 2009; 119: 3215 -22. 20. 4%

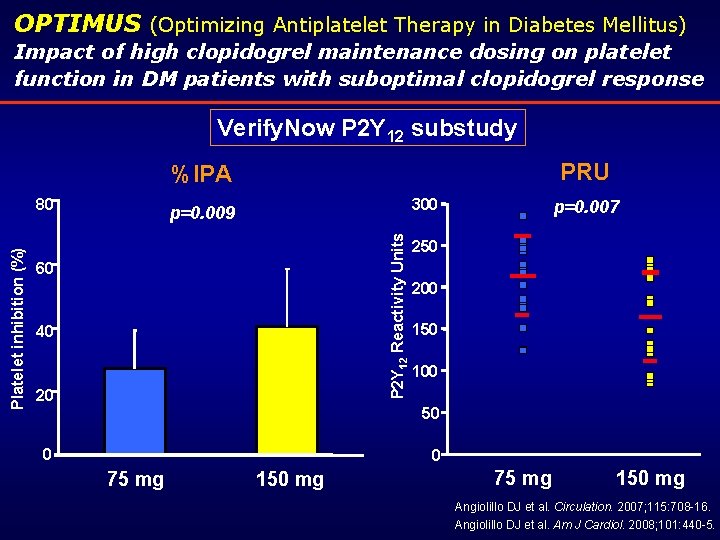
OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus) Impact of high clopidogrel maintenance dosing on platelet function in DM patients with suboptimal clopidogrel response Verify. Now P 2 Y 12 substudy PRU %IPA 300 p=0. 009 P 2 Y 12 Reactivity Units Platelet inhibition (%) 80 60 40 20 p=0. 007 250 200 150 100 50 0 0 75 mg 150 mg Angiolillo DJ et al. Circulation. 2007; 115: 708 -16. Angiolillo DJ et al. Am J Cardiol. 2008; 101: 440 -5.

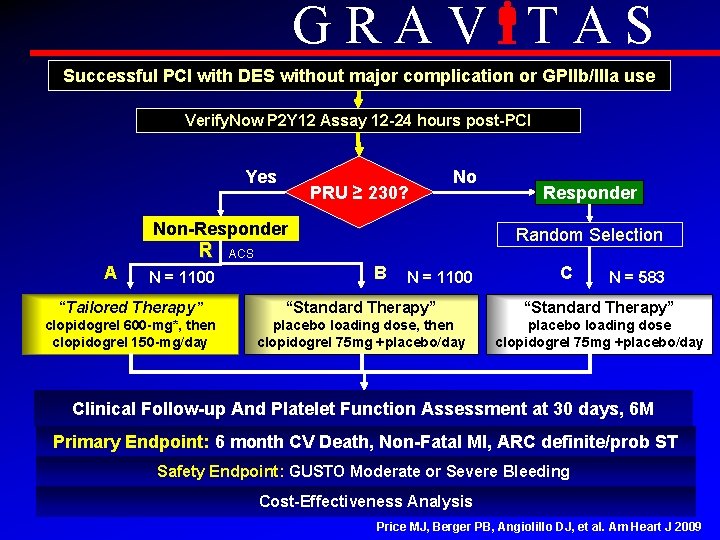
GRAV TAS Successful PCI with DES without major complication or GPIIb/IIIa use Verify. Now P 2 Y 12 Assay 12 -24 hours post-PCI Yes PRU ≥ 230? No Non-Responder R A N = 1100 Responder Random Selection ACS B N = 1100 C N = 583 “Tailored Therapy” “Standard Therapy” clopidogrel 600 -mg*, then clopidogrel 150 -mg/day placebo loading dose, then clopidogrel 75 mg +placebo/day placebo loading dose clopidogrel 75 mg +placebo/day Clinical Follow-up And Platelet Function Assessment at 30 days, 6 M Primary Endpoint: 6 month CV Death, Non-Fatal MI, ARC definite/prob ST Safety Endpoint: GUSTO Moderate or Severe Bleeding Cost-Effectiveness Analysis Price MJ, Berger PB, Angiolillo DJ, et al. Am Heart J 2009

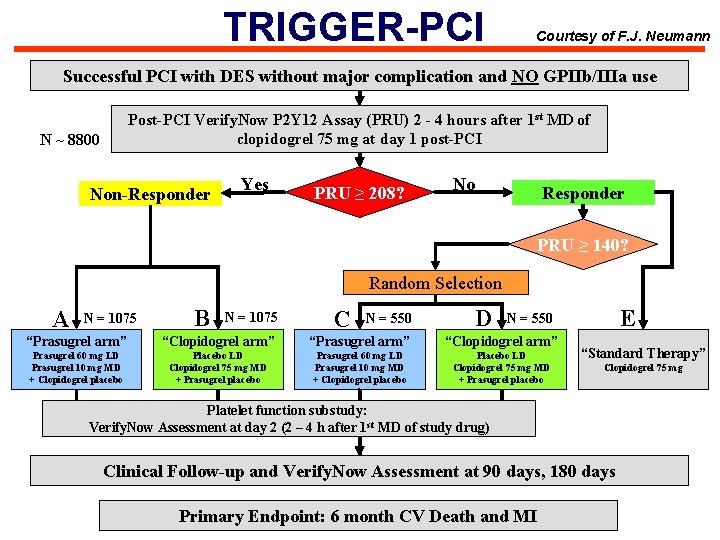
TRIGGER-PCI Courtesy of F. J. Neumann Successful PCI with DES without major complication and NO GPIIb/IIIa use Post-PCI Verify. Now P 2 Y 12 Assay (PRU) 2 - 4 hours after 1 st MD of clopidogrel 75 mg at day 1 post-PCI N ~ 8800 Non-Responder Yes PRU ≥ 208? No Responder PRU ≥ 140? Random Selection A N = 1075 B N = 1075 C N = 550 D E N = 550 “Prasugrel arm” “Clopidogrel arm” Prasugrel 60 mg LD Prasugrel 10 mg MD + Clopidogrel placebo Placebo LD Clopidogrel 75 mg MD + Prasugrel placebo “Standard Therapy” Clopidogrel 75 mg Platelet function substudy: Verify. Now Assessment at day 2 (2 – 4 h after 1 st MD of study drug) Clinical Follow-up and Verify. Now Assessment at 90 days, 180 days Primary Endpoint: 6 month CV Death and MI

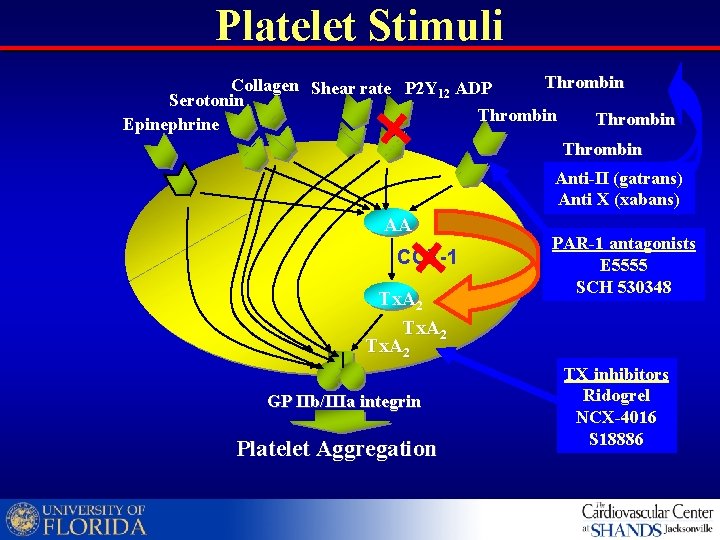
Platelet Stimuli Thrombin Collagen Shear rate P 2 Y 12 ADP Serotonin Thrombin Epinephrine Thrombin Anti-II (gatrans) Anti X (xabans) AA COX-1 Tx. A 2 GP IIb/IIIa integrin Platelet Aggregation PAR-1 antagonists E 5555 SCH 530348 TX inhibitors Ridogrel NCX-4016 S 18886

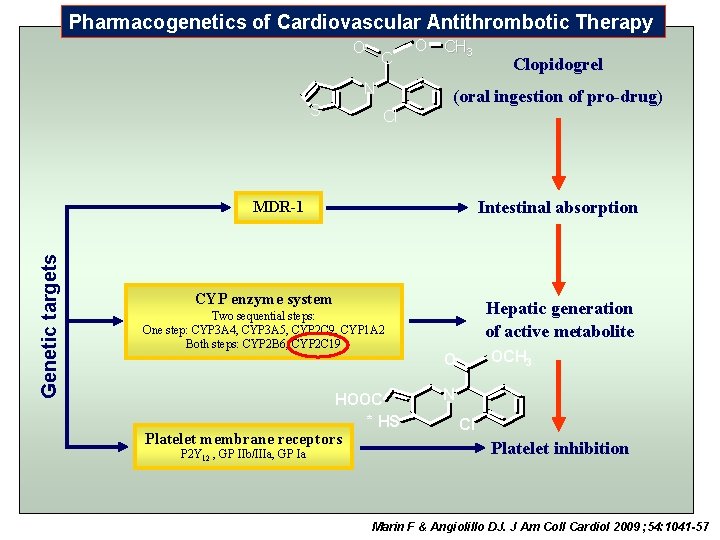
Pharmacogenetics of Cardiovascular Antithrombotic Therapy O C N S O CH 3 (oral ingestion of pro-drug) Cl Intestinal absorption Genetic targets MDR-1 CYP enzyme system Two sequential steps: One step: CYP 3 A 4, CYP 3 A 5, CYP 2 C 9, CYP 1 A 2 Both steps: CYP 2 B 6, CYP 2 C 19 HOOC * HS Platelet membrane receptors P 2 Y 12 , GP IIb/IIIa, GP Ia Clopidogrel Hepatic generation of active metabolite OCH 3 O N Cl Platelet inhibition Marin F & Angiolillo DJ. J Am Coll Cardiol 2009 ; 54: 1041 -57

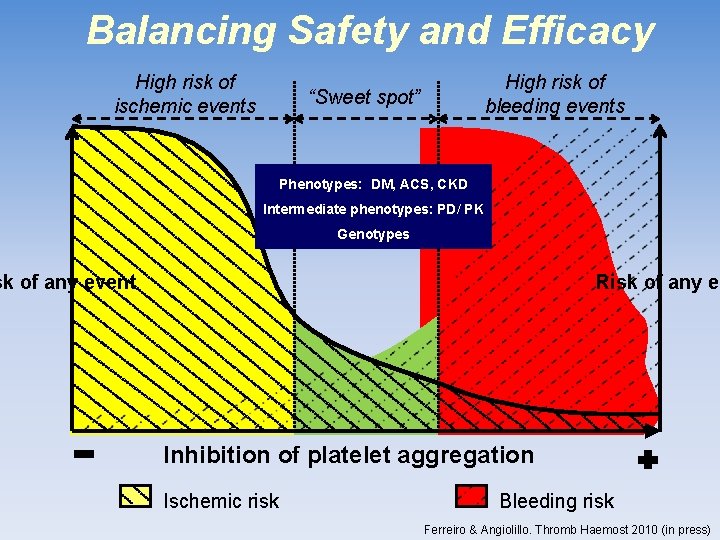
Balancing Safety and Efficacy High risk of ischemic events High risk of bleeding events “Sweet spot” Phenotypes: DM, ACS, CKD Intermediate phenotypes: PD/ PK Genotypes sk of any event Risk of any ev Inhibition of platelet aggregation Ischemic risk Bleeding risk Ferreiro & Angiolillo. Thromb Haemost 2010 (in press)

Does one size fit all? ? Need for “individualized” treatment!