PLANT TISSUE CULTURE Plant Tissue Culture Definition The













- Slides: 28

PLANT TISSUE CULTURE

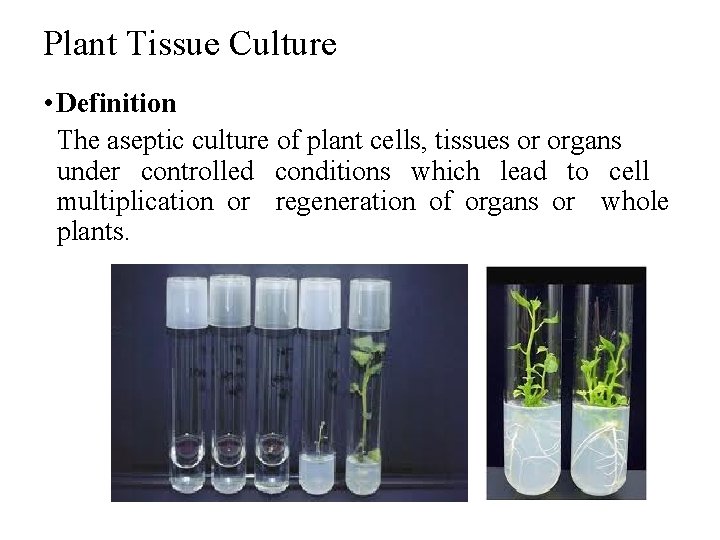
Plant Tissue Culture • Definition The aseptic culture of plant cells, tissues or organs under controlled conditions which lead to cell multiplication or regeneration of organs or whole plants.

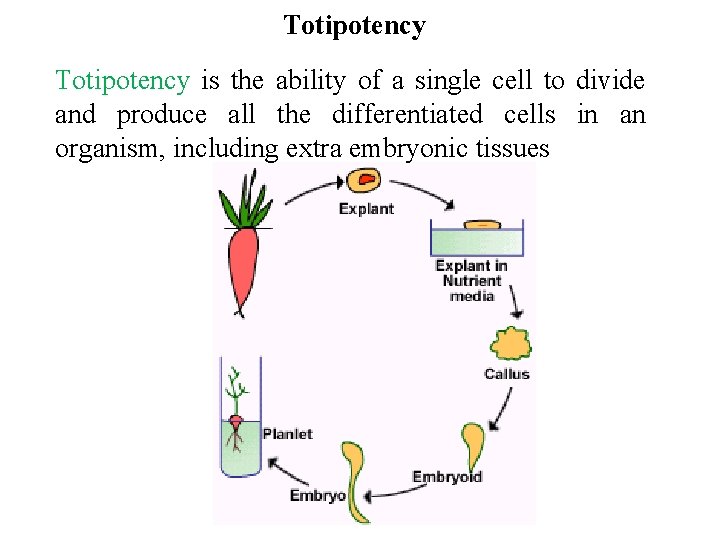
Plant Tissue Culture: History • In 1839, Schleiden and Schwann proposed that cell is the basic unit of organisms. They visualized that cell is capable of autonomy and therefore it should be possible for each cell if given an environment to regenerate into whole plant. • In 1902, German scientist Gottlieb Haberlandt introduced the term Totipotency. • Totipotency is the ability of a single cell to divide and produce all the differentiated cells in an organism, including extra embryonic tissues. • G Haberlandt is thus regarded as father of tissue culture.

Totipotency is the ability of a single cell to divide and produce all the differentiated cells in an organism, including extra embryonic tissues

• Discovery of Plant Growth Regulators In 1926, Fritz Went discovered first plant growth regulator (PGR), indoleacetic acid (IAA). IAA is a naturally occurring member of a class of PGRs termed ‘auxins’. Skoog and Tsui (1957) demonstrated induction of cell division and bud formation in tobacco by adenine. This led to further investigations by Skoog and Miller (1955) who isolated ‘kinetin’- a derivative of adenine (6 -furyl aminopurine).

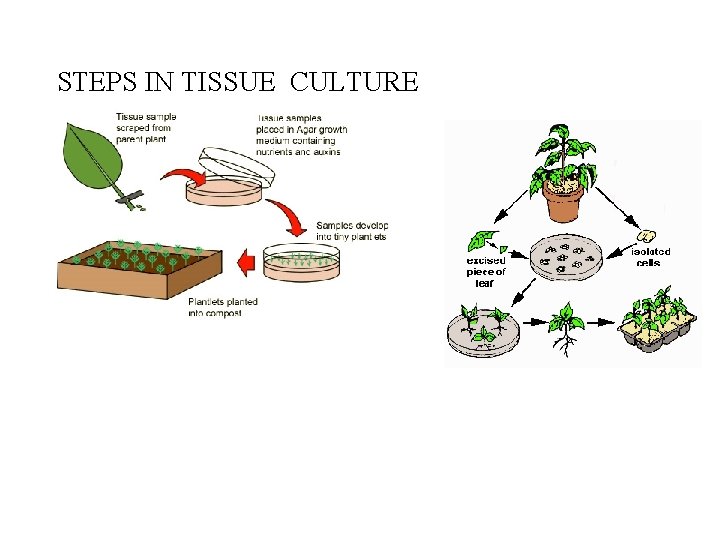
STEPS IN TISSUE CULTURE

What is needed? Tissue culture has several critical requirements: 1. Explants • A small tissue excised from any part of the plant is called explant which is the starting point. • It can be initiated from any part of plant - root, stem, petiole, leaf or flower, choice of explant varies with species. • Generally meristematic tissue or internodal segments of the plant is selected for micropropagation. • The explant must be healthy and free from obvious signs of disease or decay.

2. Growth medium/ Culture Medium Containing energy sources and inorganic salts to supply cell growth needs. This can be liquid or semisolid. The tissue so collected is placed in a culture medium/nutrient medium for the multiplication of cells. 3. Aseptic (sterile) conditions As microorganisms grow much more quickly than plant and animal tissue and can over run a culture. • Since plant cell division is slower compared to the growth of bacteria, fungi and even minor contaminants can easily overgrow the plant tissue culture. Therefore, all the materials like glassware, instruments, medium, explant etc to be used in culture work must be free of microbes.

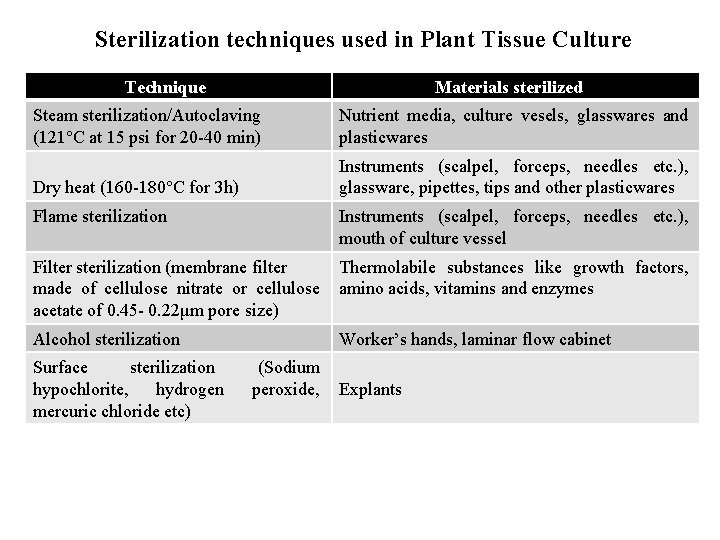
Sterilization techniques used in Plant Tissue Culture Technique Materials sterilized Steam sterilization/Autoclaving (121°C at 15 psi for 20 -40 min) Nutrient media, culture vesels, glasswares and plasticwares Dry heat (160 -180°C for 3 h) Instruments (scalpel, forceps, needles etc. ), glassware, pipettes, tips and other plasticwares Flame sterilization Instruments (scalpel, forceps, needles etc. ), mouth of culture vessel Filter sterilization (membrane filter made of cellulose nitrate or cellulose acetate of 0. 45 - 0. 22μm pore size) Thermolabile substances like growth factors, amino acids, vitamins and enzymes Alcohol sterilization Worker’s hands, laminar flow cabinet Surface sterilization hypochlorite, hydrogen mercuric chloride etc) (Sodium peroxide, Explants

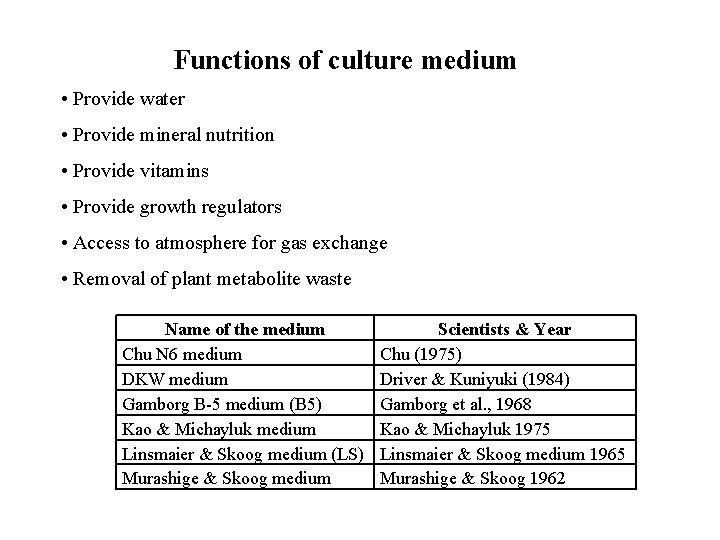
Functions of culture medium • Provide water • Provide mineral nutrition • Provide vitamins • Provide growth regulators • Access to atmosphere for gas exchange • Removal of plant metabolite waste Name of the medium Chu N 6 medium DKW medium Gamborg B-5 medium (B 5) Kao & Michayluk medium Linsmaier & Skoog medium (LS) Murashige & Skoog medium Scientists & Year Chu (1975) Driver & Kuniyuki (1984) Gamborg et al. , 1968 Kao & Michayluk 1975 Linsmaier & Skoog medium 1965 Murashige & Skoog 1962

Components of tissue culture media 1. 2. 3. 4. 5. 6. Inorganic Nutrients Carbon Source Organic Supplements Plant growth Hormones (PGR’s) Solidifying agents p. H

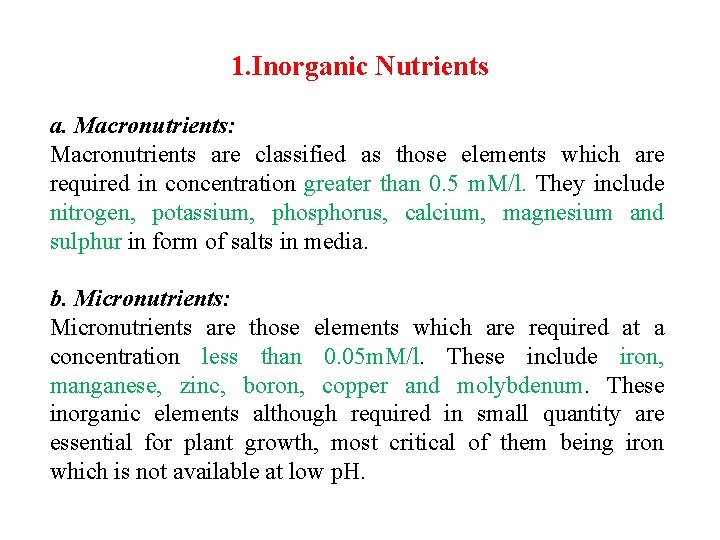
1. Inorganic Nutrients a. Macronutrients: Macronutrients are classified as those elements which are required in concentration greater than 0. 5 m. M/l. They include nitrogen, potassium, phosphorus, calcium, magnesium and sulphur in form of salts in media. b. Micronutrients: Micronutrients are those elements which are required at a concentration less than 0. 05 m. M/l. These include iron, manganese, zinc, boron, copper and molybdenum. These inorganic elements although required in small quantity are essential for plant growth, most critical of them being iron which is not available at low p. H.

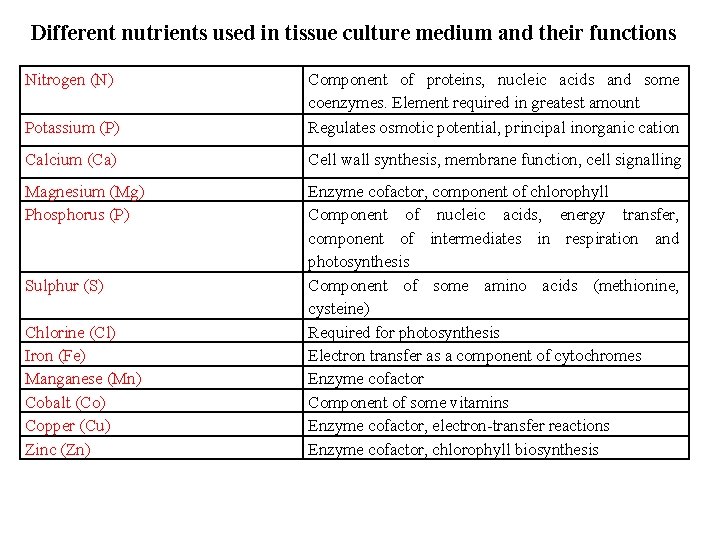
Different nutrients used in tissue culture medium and their functions Nitrogen (N) Potassium (P) Component of proteins, nucleic acids and some coenzymes. Element required in greatest amount Regulates osmotic potential, principal inorganic cation Calcium (Ca) Cell wall synthesis, membrane function, cell signalling Magnesium (Mg) Phosphorus (P) Enzyme cofactor, component of chlorophyll Component of nucleic acids, energy transfer, component of intermediates in respiration and photosynthesis Component of some amino acids (methionine, cysteine) Required for photosynthesis Electron transfer as a component of cytochromes Enzyme cofactor Component of some vitamins Enzyme cofactor, electron-transfer reactions Enzyme cofactor, chlorophyll biosynthesis Sulphur (S) Chlorine (Cl) Iron (Fe) Manganese (Mn) Cobalt (Co) Copper (Cu) Zinc (Zn)

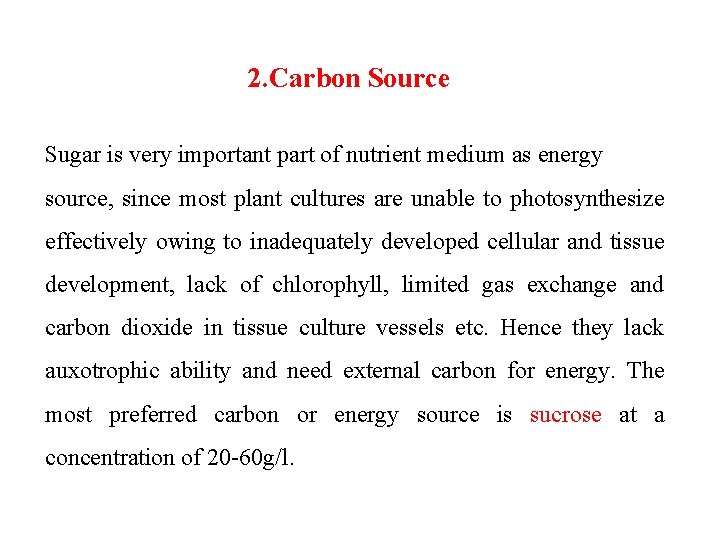
2. Carbon Source Sugar is very important part of nutrient medium as energy source, since most plant cultures are unable to photosynthesize effectively owing to inadequately developed cellular and tissue development, lack of chlorophyll, limited gas exchange and carbon dioxide in tissue culture vessels etc. Hence they lack auxotrophic ability and need external carbon for energy. The most preferred carbon or energy source is sucrose at a concentration of 20 -60 g/l.

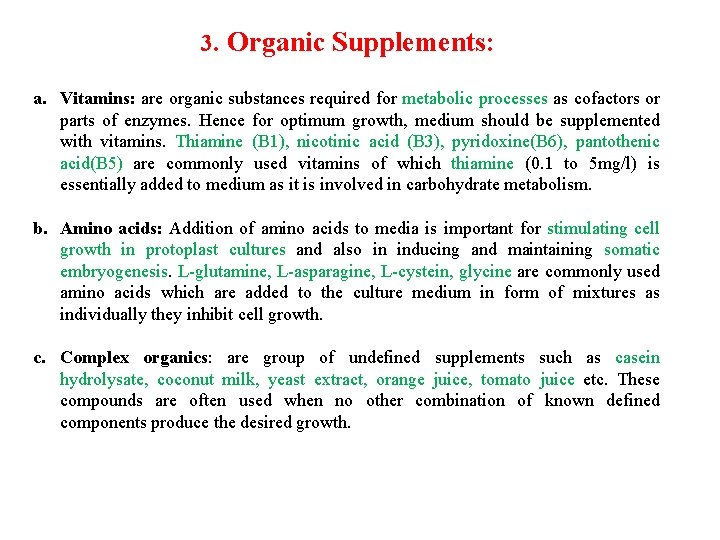
3. Organic Supplements: a. Vitamins: are organic substances required for metabolic processes as cofactors or parts of enzymes. Hence for optimum growth, medium should be supplemented with vitamins. Thiamine (B 1), nicotinic acid (B 3), pyridoxine(B 6), pantothenic acid(B 5) are commonly used vitamins of which thiamine (0. 1 to 5 mg/l) is essentially added to medium as it is involved in carbohydrate metabolism. b. Amino acids: Addition of amino acids to media is important for stimulating cell growth in protoplast cultures and also in inducing and maintaining somatic embryogenesis. L-glutamine, L-asparagine, L-cystein, glycine are commonly used amino acids which are added to the culture medium in form of mixtures as individually they inhibit cell growth. c. Complex organics: are group of undefined supplements such as casein hydrolysate, coconut milk, yeast extract, orange juice, tomato juice etc. These compounds are often used when no other combination of known defined components produce the desired growth.

4. Plant Growth Regulators (PGRs) Stimulate cell division and hence regulate the growth and differentiation of shoot and roots on explants and embryos in semisolid or in liquid medium cultures. The four major PGRs used are auxins, cytokinin, gibberellins and abscissic acid and their addition is must to the culture medium. a. Auxins: induce cell division, cell elongation, apical dominance, adventitious root formation, somatic embryogenesis. When used in low concentration, auxins induce root initiation and in high, callus formation occurs. Commonly used synthetic auxins are 1 -naphthaleneacetic acid (NAA). 2, 4 dichlorophenoxyacetic acid (2, 4 -D), indole-3 acetic acid (IAA), indolebutyric acid (IBA) etc. b. Cytokinins: promote cell division and stimulate initiation and growth of shoots in vitro. Zeatin, 6 - benzylaminopurine (BAP), kinetin, 2 -i. P are the frequently used cytokinins. They modify apical dominance by promoting axillary shoot formation. When used in high concentration, CK inhibits root formation and induces adventitious shoot formation. The ratio of auxin and cytokinin in the culture decides morphogenesis.

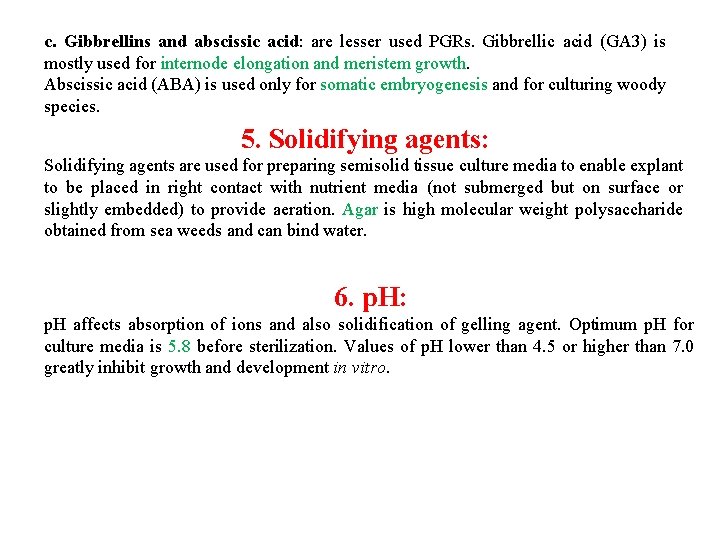
c. Gibbrellins and abscissic acid: are lesser used PGRs. Gibbrellic acid (GA 3) is mostly used for internode elongation and meristem growth. Abscissic acid (ABA) is used only for somatic embryogenesis and for culturing woody species. 5. Solidifying agents: Solidifying agents are used for preparing semisolid tissue culture media to enable explant to be placed in right contact with nutrient media (not submerged but on surface or slightly embedded) to provide aeration. Agar is high molecular weight polysaccharide obtained from sea weeds and can bind water. 6. p. H: p. H affects absorption of ions and also solidification of gelling agent. Optimum p. H for culture media is 5. 8 before sterilization. Values of p. H lower than 4. 5 or higher than 7. 0 greatly inhibit growth and development in vitro.

CALLUS • THE CULTURED TISSUE WILL GROW INTO AN UNORGANISED MASS CALLED AS CALLUS. • AS THE CALLUS GROWS THE NUTRIENTS IN THE MEDIUM STARTS DEPLETING. • THE CALLUS SHOULD BE PETRIODICALLY CHANGED INTO NEW CULTURE MEDIUM FOR HEALTHY GROWTH OF THE PLANTS.

Steps involved in the in vitro micropropagation Cleaning of glassware Preparation of nutrient medium Selection and sterilization of explant. Inoculation of aseptic explant in to nutrient medium. Proliferation of shoots on a multiplication medium. Transfer of shoots for sub-culturing. Rooting and hardening of plantlets Field trials.

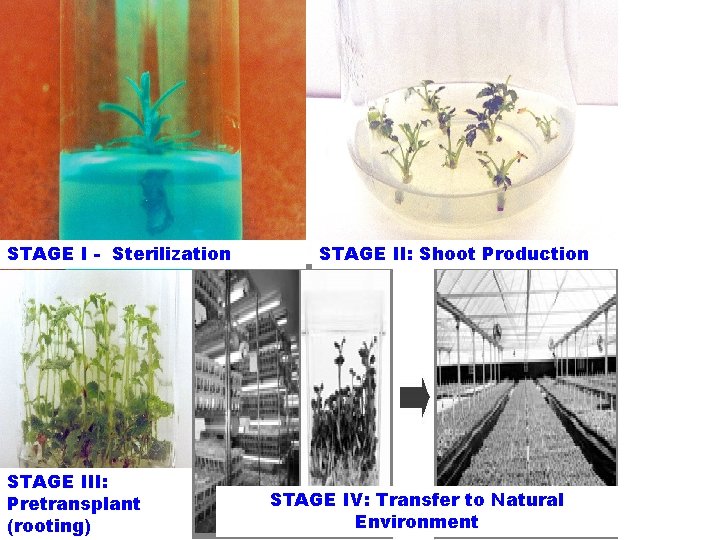
Stages of micropropagation STAGE I - Sterilization STAGE III: Pretransplant (rooting) STAGE II: Shoot Production STAGE IV: Transfer to Natural Environment

Culturing (micropropagating) Plant Tissue - the steps • Selection of the plant tissue (explant) from a healthy vigorous ‘mother plant’ - this is often the apical bud, but can be other tissue. • This tissue must be sterilized to remove microbial contaminants ü ü Alcohol Calcium hypochlorite Mercuric chloride Hydrogen peroxide

The Steps, II Shoot Production • Establishment of the explant in a culture medium. The medium sustains the plant cells and encourages cell division. It can be solid or liquid • Each plant species (and sometimes the variety within a species) has particular medium requirements that must be established by trial and error

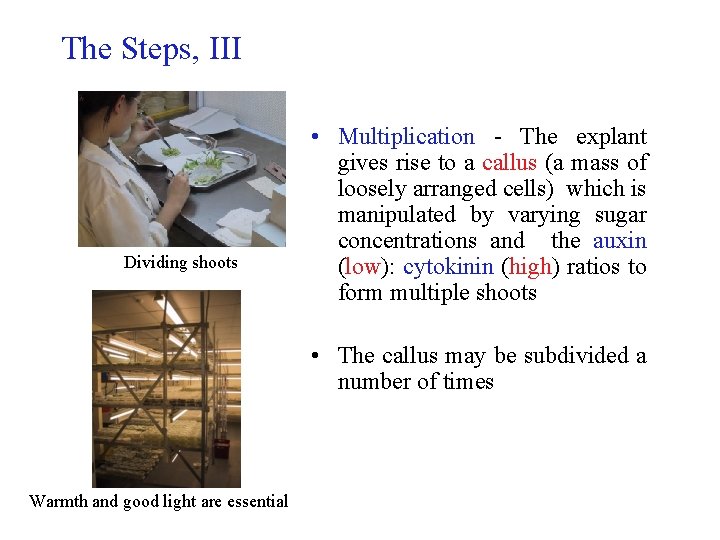
The Steps, III Dividing shoots • Multiplication - The explant gives rise to a callus (a mass of loosely arranged cells) which is manipulated by varying sugar concentrations and the auxin (low): cytokinin (high) ratios to form multiple shoots • The callus may be subdivided a number of times Warmth and good light are essential

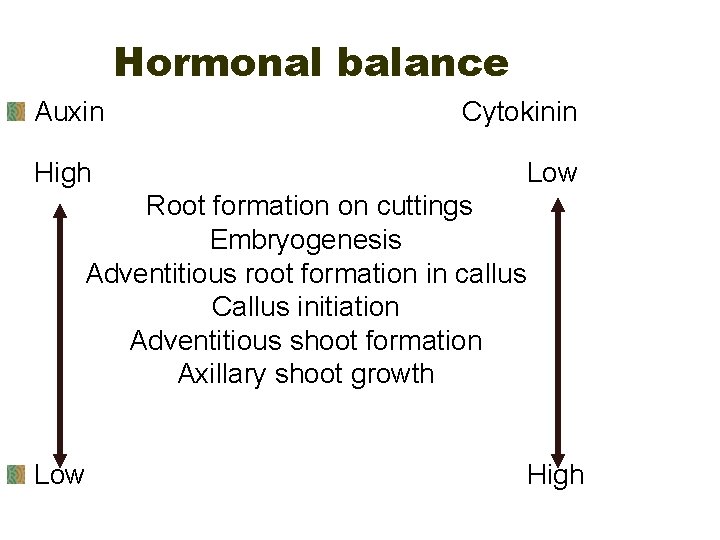
Hormonal balance Auxin Cytokinin High Low Root formation on cuttings Embryogenesis Adventitious root formation in callus Callus initiation Adventitious shoot formation Axillary shoot growth Low High

The Steps, IV • Root formation - The shoots are transferred to a growth medium with relatively higher auxin: cytokinin ratios The bottles on these racks are young banana plants and are growing roots

The Steps, V • The rooted shoots potted up (deflasked) ‘hardened off’ gradually decreasing humidity are and by the • This is necessary as many young tissue culture plants have no waxy cuticle to prevent water loss Tissue culture plants sold to a nursery & then potted up


Advantages of in tissue culture üProduction of secondary metabolites: Plants being important source of variety of chemicals used in pharmacy, medicine and industry, cell cultures are effectively utilized for production of these chemicals on a commercial scale for enhanced yield and better production control. üTo produce plants anytime, although the climates are not appropriate to produce a plant. Moreover, if seed is not available, it is possible to produce a plant with this method. üIf there is plant with partially infected tissue, it is possible to produce a new plant without infection. üTo produce high yield crops üSpace-saving--little space needs üPlants are free of pests, pathogens and viruses üLabor-saving---no transfer labor (under storage conditions) üStored cultures can be used as nuclear stock for vegetative preservation üInternational shipping restrictions are lessened • no soil • pest-free plants