Plant Tissue Culture An excellent biological tool for

Plant Tissue Culture An excellent biological tool for conservation and sustainable use of bio-resources

Sustainable use of bioresources Sustainable use of plant is defined as 'the level of harvest at which a species can maintain its population at natural or near-natural levels and the harvest, will not change the species composition of the community’ Conserving an ecological balance by avoiding depletion of natural resources. Capable of being continued with minimal longterm effect on the environment

Plant Tissue Culture Plant tissue culture can be defined as the culture of plant cells, tissue and organs under aseptic conditions

TOTIPOTENCY Haberlandt 1902: Totipotency - genetic potential of cells to undergo embryogenesis; now extended to include organogenesis. Haberlandt proposed that all cells are totipotent.
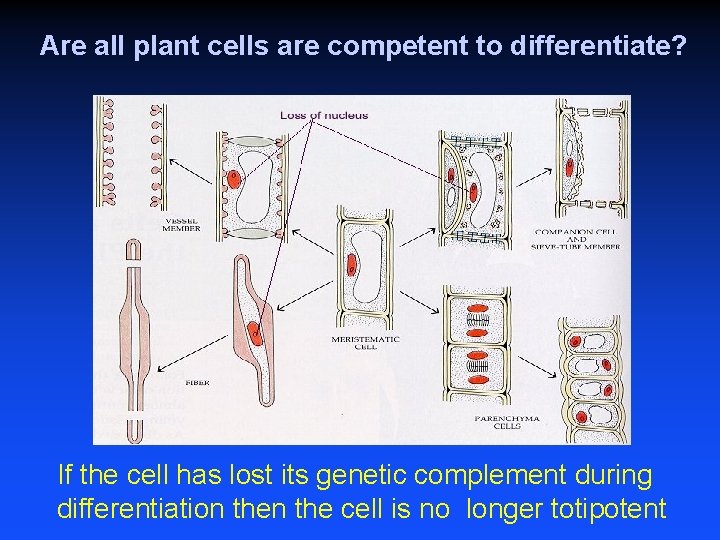
Are all plant cells are competent to differentiate? If the cell has lost its genetic complement during differentiation the cell is no longer totipotent
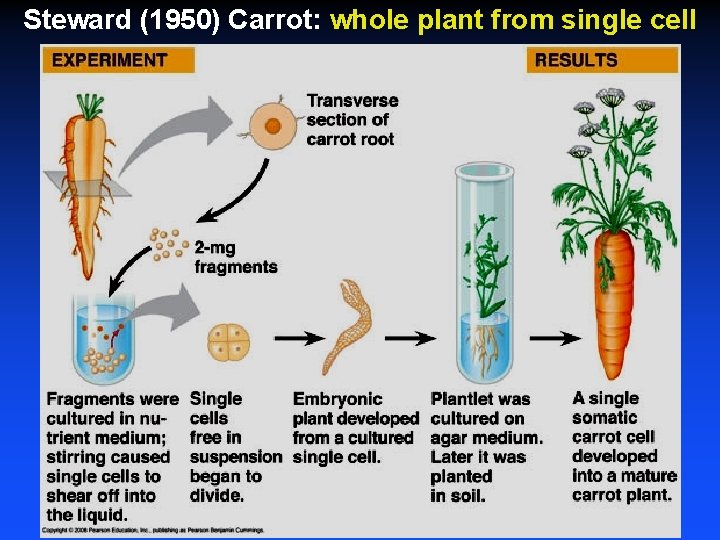
Steward (1950) Carrot: whole plant from single cell
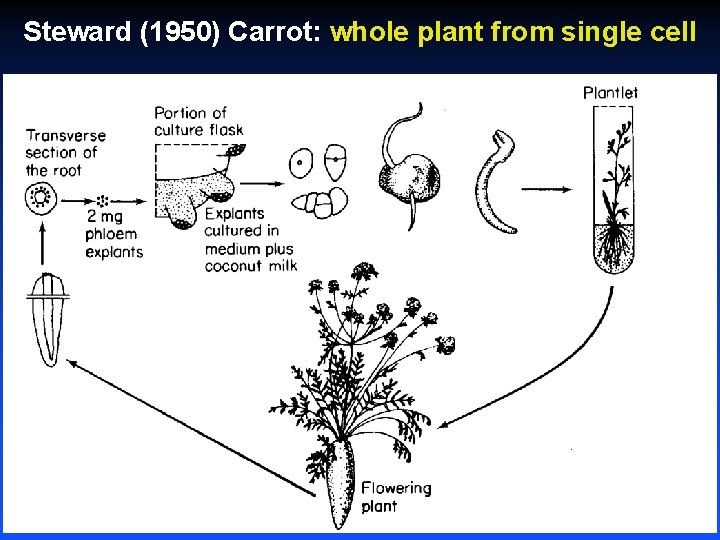
Steward (1950) Carrot: whole plant from single cell

The great luminaries Haberlandt 1902 Idea ! First in vitro cultivation of plant tissues: 1939 Cocking (1960) Protoplast Isolation and culture Murashige and Skoog medium (1962) Melchers (1978) Fusion of potato and tomato protoplasts

The great luminaries Haberlandt 1902 The father of plant tissue culture German Botanist HABERLANDT who conceived concept of cell culture First in vitro cultivationthe of plant tissues: 1939 Cocking (1960) in 1902. He cultured pallisade cells, Protoplast pith cells, stamen hairs and stomatal Isolation and culture guard cells in a simple organically enriched medium containing glucose under aseptic conditions and was totally unsuccessful in all cases. Murashige and Skoog medium (1962) Melchers (1978) Fusion of potato and tomato protoplasts

The great luminaries Haberlandt 1902 Idea ! First in vitro cultivation of plant tissues: 1939 Cocking (1960) Protoplast The in vitro cultivation of plant tissues for indefinite Isolation and culture periods of time was achieved by: • WHITE (1934) established medium & cultured tomato roots, • WHITE (1939) cultured tissues of Nicotiana hybrid Murashige Skoog medium (1962) (Nicotiana glauca andand. N. langsdorffii) and • GAUTHERET and NOBECOURT (1939) established Melchers (1978) unlimited growth of roots of carrot Fusion using IAA. of potato and tomato protoplasts
The great luminaries E. C. Cocking developed the method of enzymatic isolation and culture of Idea ! First in vitroremoving cultivation of plant tissues: 1939 protoplasts by the cell wall with cellulase and pectinase and regulating protoplast expansion with an external osmoticum (Cocking, 1960). Haberlandt 1902 Murashige and Skoog medium (1962) Melchers (1978) Fusion of potato and tomato protoplasts
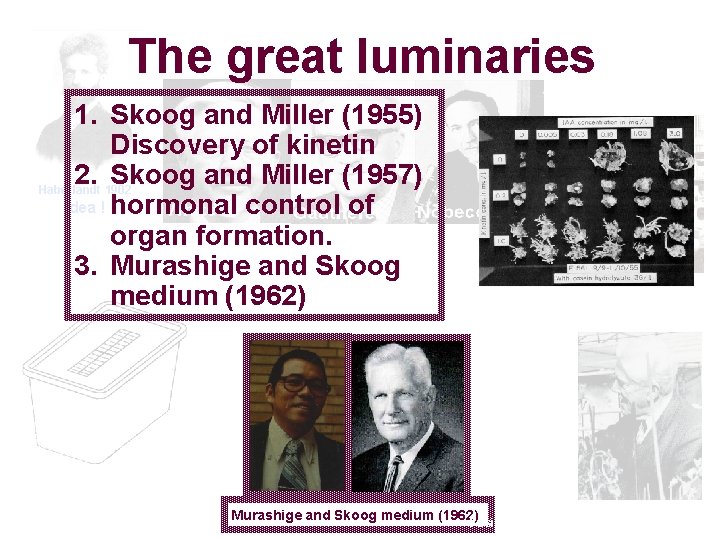
The great luminaries 1. Skoog and Miller (1955) Discovery of kinetin 2. Skoog and Miller (1957) Haberlandt 1902 Idea ! hormonal control of Firstformation. in vitro cultivation of plant tissues: 1939 organ 3. Murashige and Skoog medium (1962) Melchers (1978) Fusion of potato and tomato protoplasts Murashige and Skoog medium (1962)

The great luminaries Melchers (1978) Melchers and his co-workers Idea ! (1978) First produced a hybrid plant in vitro cultivation of plant tissues: 1939 from the fusion of potato and tomato protoplasts. The method provides the opportunity of producing hybrids between sexually incompatible species. Haberlandt 1902 Murashige and Skoog medium (1962)
Plant Tissue Culture • Plant tissue culture can be defined as the culture of plant cells, tissue and organs under aseptic conditions
Plant Cell, Tissue, and Organ Culture Key Parameters for Manipulation of Plant Cell, Tissue and Organ Cultures 1. Nutrient Media 2. Culture Explants 3. Culture Growth Environments Modulating these factors/components is the basis for successful culturing, including regulating growth and development.

1. Nutrient Media I. Inorganic salts/mineral nutrients A. Composition, essential micro- and macronutrients* B. Quantity and form of nutrient C. Optimizing formulations II. Organic constituents A. Carbon source* B. Growth regulators* C. Vitamins D. Hexitols E. Others III. Natural complexes IV. Physical support agents V. Media preparation *Basal/essential constituents of all (most) media

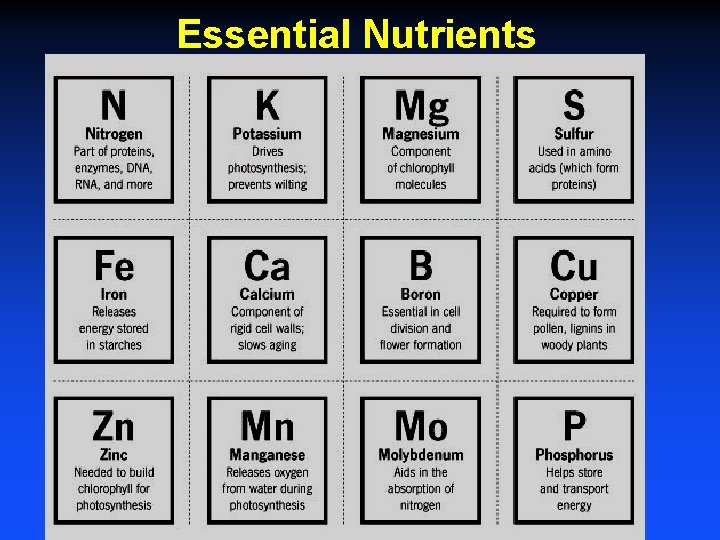
Essential Nutrients Macronutrients (required content in the plant - 0. 1% or % per dry weight) - C, H, O, P, K, N, S, Ca, Mg Micronutrients (requirement - ppm/dry weight) Fe, Mn, Zn, Cu, B, Cl, Mo Na, Se and Si are essential for some plants
Essential Nutrients
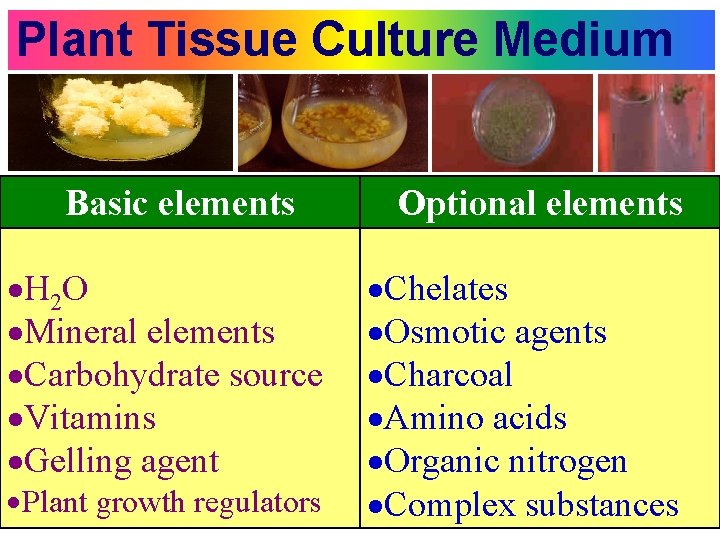
Plant Tissue Culture Medium Basic elements H 2 O Mineral elements Carbohydrate source Vitamins Gelling agent Plant growth regulators Optional elements Chelates Osmotic agents Charcoal Amino acids Organic nitrogen Complex substances
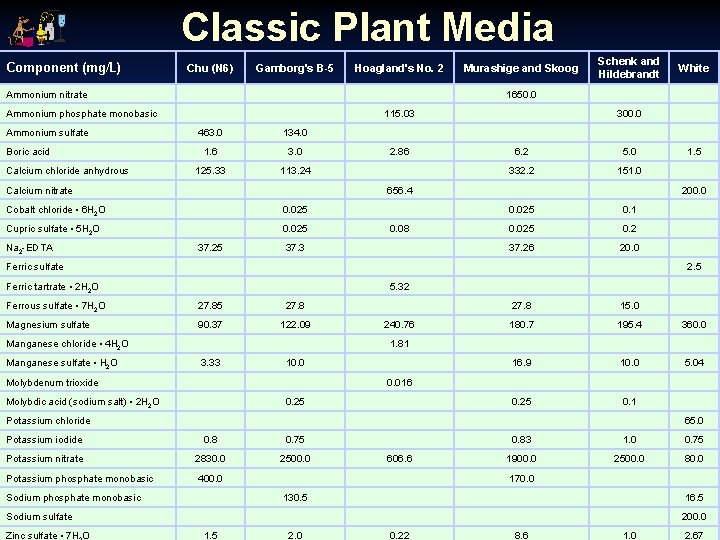
Classic Plant Media Component (mg/L) Chu (N 6) Gamborg's B-5 Hoagland's No. 2 Ammonium nitrate Boric acid Calcium chloride anhydrous 115. 03 463. 0 134. 0 1. 6 3. 0 125. 33 113. 24 Calcium nitrate 0. 025 Cupric sulfate • 5 H 2 O 0. 025 2. 86 6. 2 5. 0 332. 2 151. 0 37. 25 0. 08 37. 3 0. 025 0. 1 0. 025 0. 2 37. 26 20. 0 2. 5 Ferric tartrate • 2 H 2 O 5. 32 Ferrous sulfate • 7 H 2 O 27. 85 27. 8 Magnesium sulfate 90. 37 122. 09 Manganese chloride • 4 H 2 O 240. 76 27. 8 15. 0 180. 7 195. 4 360. 0 16. 9 10. 0 5. 04 0. 25 0. 1 1. 81 3. 33 10. 0 Molybdenum trioxide 0. 016 Molybdic acid (sodium salt) • 2 H 2 O 0. 25 Potassium chloride 65. 0 Potassium iodide 0. 8 0. 75 Potassium nitrate 2830. 0 2500. 0 Potassium phosphate monobasic 400. 0 Sodium phosphate monobasic 606. 6 0. 83 1. 0 0. 75 1900. 0 2500. 0 80. 0 170. 0 130. 5 16. 5 Sodium sulfate Zinc sulfate • 7 H O 1. 5 200. 0 Ferric sulfate Manganese sulfate • H 2 O White 300. 0 656. 4 Cobalt chloride • 6 H 2 O Na 2 -EDTA Schenk and Hildebrandt 1650. 0 Ammonium phosphate monobasic Ammonium sulfate Murashige and Skoog 200. 0 1. 5 2. 0 0. 22 8. 6 1. 0 2. 67
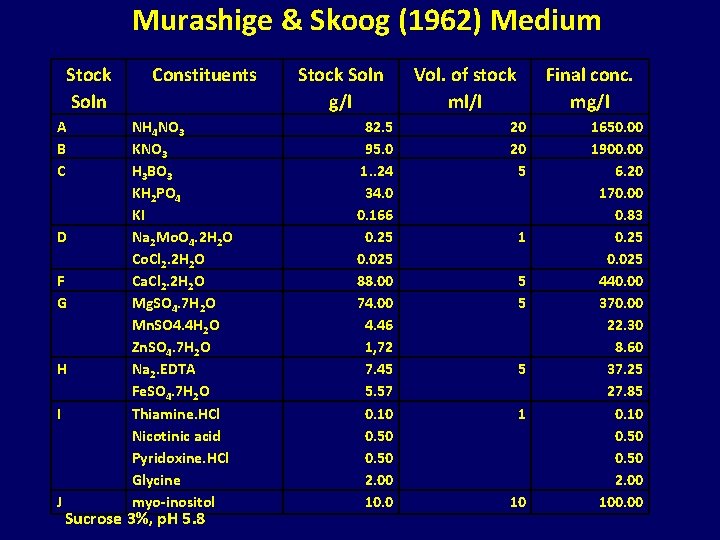
Murashige & Skoog (1962) Medium Stock Soln A B C D F G H I J Constituents NH 4 NO 3 KNO 3 H 3 BO 3 KH 2 PO 4 KI Na 2 Mo. O 4. 2 H 2 O Co. Cl 2. 2 H 2 O Ca. Cl 2. 2 H 2 O Mg. SO 4. 7 H 2 O Mn. SO 4. 4 H 2 O Zn. SO 4. 7 H 2 O Na 2. EDTA Fe. SO 4. 7 H 2 O Thiamine. HCl Nicotinic acid Pyridoxine. HCl Glycine myo-inositol Sucrose 3%, p. H 5. 8 Stock Soln g/l 82. 5 95. 0 1. . 24 34. 0 0. 166 0. 25 0. 025 88. 00 74. 00 4. 46 1, 72 7. 45 5. 57 0. 10 0. 50 2. 00 10. 0 Vol. of stock ml/l Final conc. mg/l 20 20 5 1 5 5 5 1 10 1650. 00 1900. 00 6. 20 170. 00 0. 83 0. 25 0. 025 440. 00 370. 00 22. 30 8. 60 37. 25 27. 85 0. 10 0. 50 2. 00 100. 00

Optimizing salt formulations - p. H, chemical stability, physiological considerations Nitrogen form - e. g. NH 4+ stimulates organogenesis and NO 3 embryogenesis of carrot callus, affects p. H and root initiation (NH 4+ - p. H , NO 3 - - p. H ), see example Iron stability - chelated forms are more chemically stable in the medium than unchelated forms K+ absorption - competitively inhibited by Na+ and this inhibition is reduced by Ca 2+
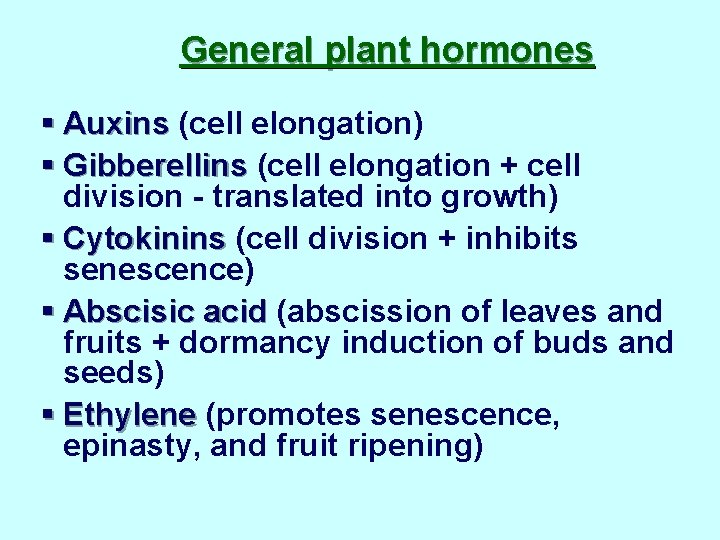
General plant hormones § Auxins (cell elongation) § Gibberellins (cell elongation + cell division - translated into growth) § Cytokinins (cell division + inhibits senescence) § Abscisic acid (abscission of leaves and fruits + dormancy induction of buds and seeds) § Ethylene (promotes senescence, epinasty, and fruit ripening)

Medium preparation collecting the ingredients mixing the ingredients, setting p. H, warming up filling the vessels autoclaving
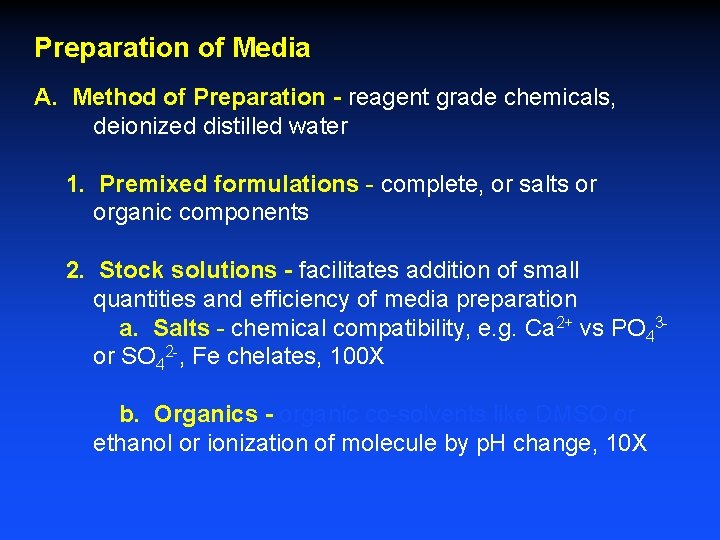
Preparation of Media A. Method of Preparation - reagent grade chemicals, deionized distilled water 1. Premixed formulations - complete, or salts or organic components 2. Stock solutions - facilitates addition of small quantities and efficiency of media preparation a. Salts - chemical compatibility, e. g. Ca 2+ vs PO 43 or SO 42 -, Fe chelates, 100 X b. Organics - organic co-solvents like DMSO or ethanol or ionization of molecule by p. H change, 10 X

Preparation of Media - contd. B. p. H of Nutrient Media - p. H may be 5. 0 to 6. 0 at start but can vary from 4. 0 to 6. 0 during the culture period and this is affected by the components in the medium. p. H influences on plant material or chemical stability of medium components C. Quantity of Medium - minimum density requirement and tissue mass gain correlates with inoculum size

Sterilization of Media 1. Thermal sterilization - 121 C, 15 lbs/in 2, 15 to 20 min for 2 L volume, most common components of plant tissue culture media are relatively heat stable; notable exceptions are reducing sugars (glucose and fructose) and antibiotics; Considerations: Reducing sugars – interactions with amino acids/salts Amino acids – inactivation by interaction with sugars/Maillard reaction Growth regulators – all stable enough biologically for autoclave sterilization, however, gibberellins are chemically unstable 2. Filter sterilization - 0. 22 or 0. 45 m mesh membranes, antibiotics 3. Radiosterilization - gamma irradiation 4. Gas sterilization - ethylene oxide There are instances when chemical stability and biological activity are not correlated

Preparation and Culture of Explants Explant - portion of a plant, organ or tissue that is inoculated into culture, choice of explant typically is based on the type of growth or differentiation that is desired I. Elimination of microbial contaminants A. Surface contaminants - principally microbial saprophytes that are eliminated by surface disinfestation B. Internal contaminants - principally pathogens that are eliminated by thermotherapy (35 -40 C) and culture of explants free of organisms or by antibiotics II. Maintenance of asepsis (free from microorganisms) during excision and culture - procedures are carried out in sterile laminar flow positive pressure hoods (0. 3 m HEPA filters)

3. CULTURE ENVIRONMENT I. Temperature – Vary, genotype dependent A. Absolute - 22 -28°C B. Constant, diurnal C. Seasonal II. Illumination A. Quality - roots - red light and shoots - UV and blue light B. Intensity - low light intensity, 1000 lux or 20 E m-1 s-2 C. Photoperiod - 16 hours/daily III. Humidity Too high - contamination, too low - medium dehydration IV. Atmospheric gases Little is known except for CO 2 for photoautotrophic cells, tissue, etc. Head space gases may affect growth and development
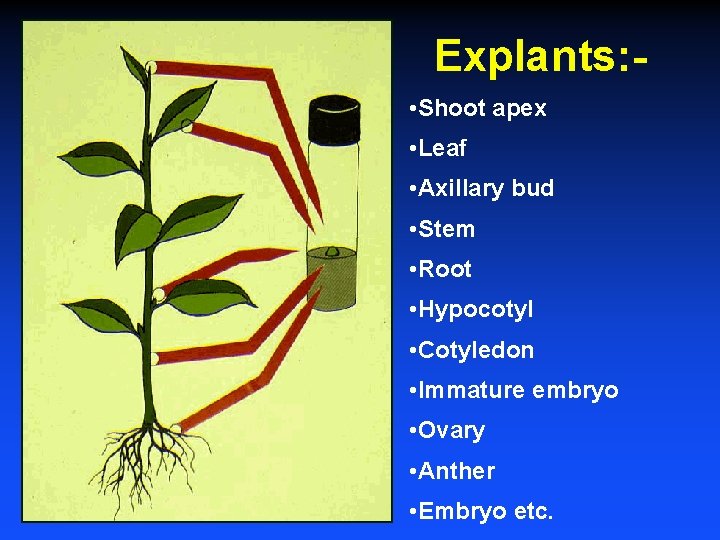
Explants: • Shoot apex • Leaf • Axillary bud • Stem • Root • Hypocotyl • Cotyledon • Immature embryo • Ovary • Anther • Embryo etc.
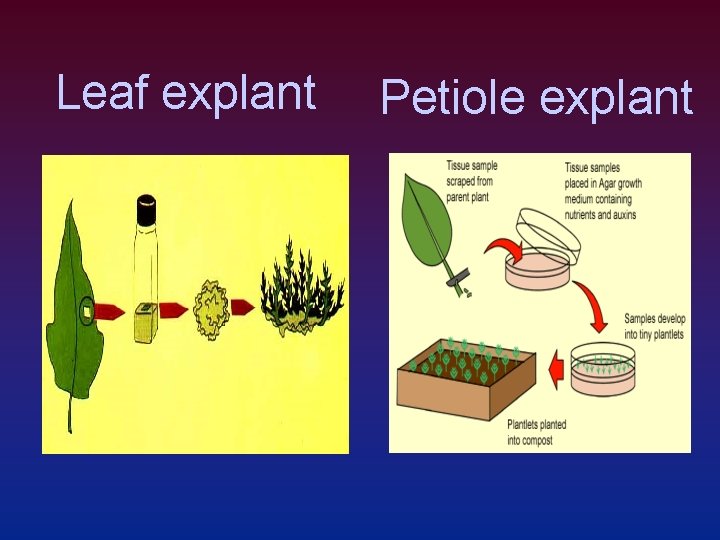
Leaf explant Petiole explant
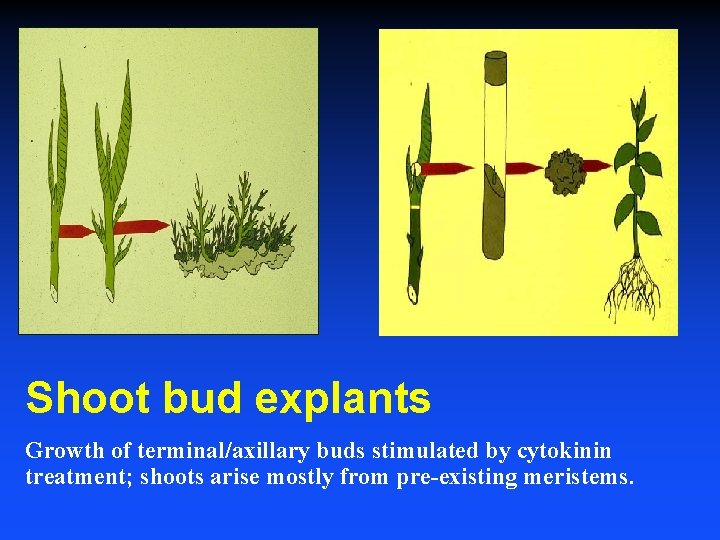
Shoot bud explants Growth of terminal/axillary buds stimulated by cytokinin treatment; shoots arise mostly from pre-existing meristems.
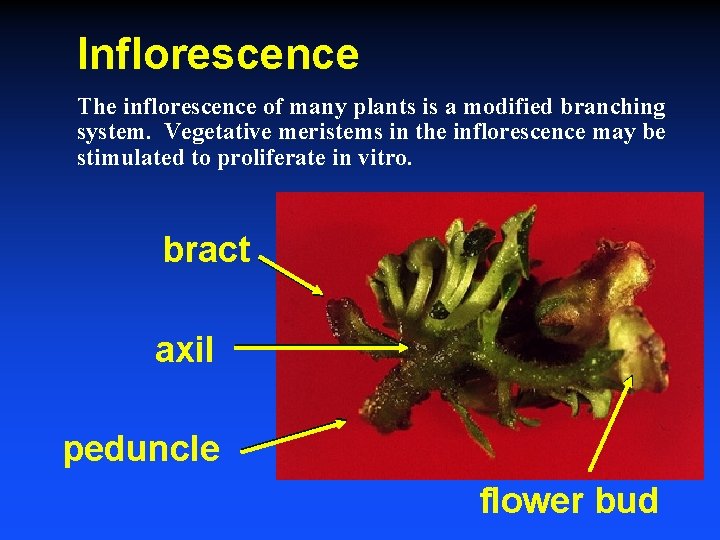
Inflorescence The inflorescence of many plants is a modified branching system. Vegetative meristems in the inflorescence may be stimulated to proliferate in vitro. bract axil peduncle flower bud
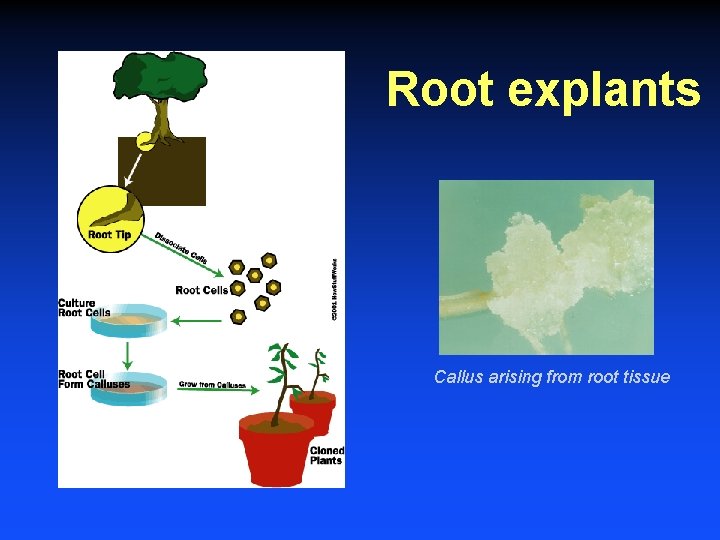
Root explants Callus arising from root tissue
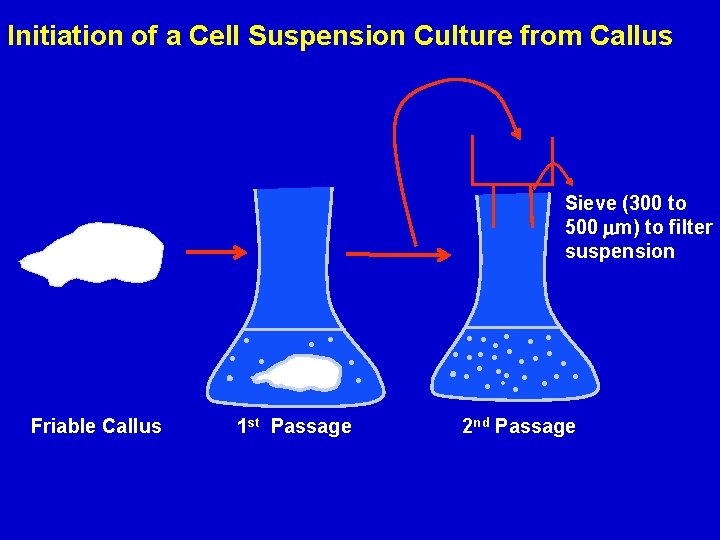
Initiation of a Cell Suspension Culture from Callus Sieve (300 to 500 m) to filter suspension Friable Callus 1 st Passage 2 nd Passage

Inoculation of explants

Aseptic Inoculation
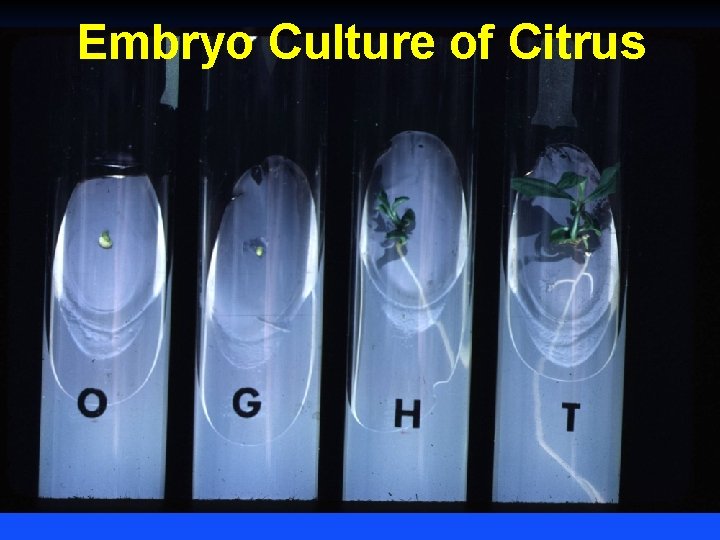
Embryo Culture of Citrus

Culture chambers
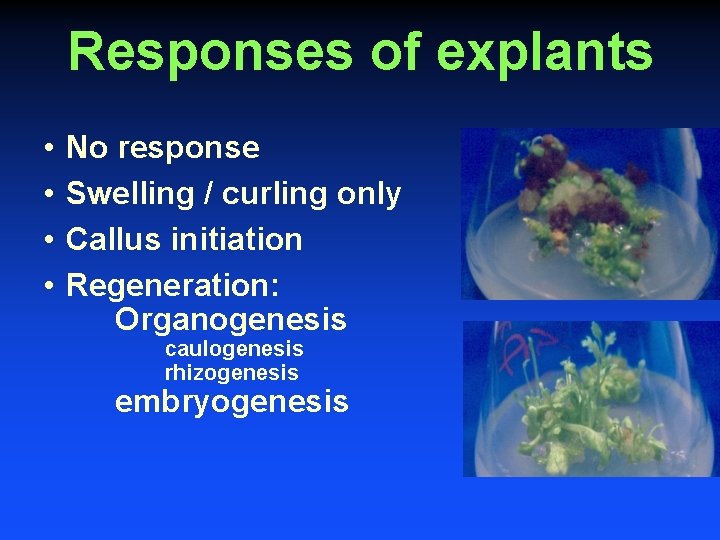
Responses of explants • • No response Swelling / curling only Callus initiation Regeneration: Organogenesis caulogenesis rhizogenesis embryogenesis
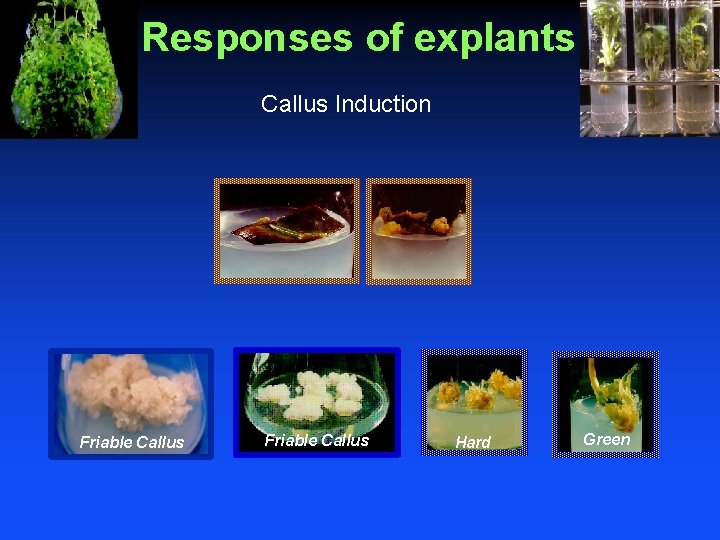
Responses of explants Callus Induction Friable Callus Hard Green

Responses of explants Organogenesis caulogenesis Multiple shoots Rhizogenesis shoots and roots Embryogenesis roots and shoots Rhizogenesis

Experimental morphogenesis Sucrose level variation 0% Sucrose 0. 5 mg. l-1 NAA 0. 5 mg. l-1 BAP 1% Sucrose 0. 5 mg. l-1 NAA 0. 5 mg. l-1 BAP 3% Sucrose 0. 5 mg. l-1 NAA 0. 5 mg. l-1 BAP 5% Sucrose 0. 5 mg. l-1 NAA 0. 5 mg. l-1 BAP
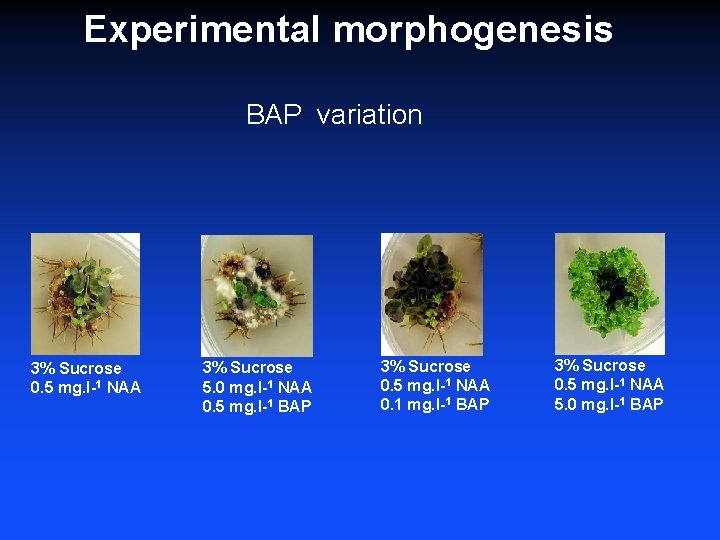
Experimental morphogenesis BAP variation 3% Sucrose 0. 5 mg. l-1 NAA 3% Sucrose 5. 0 mg. l-1 NAA 0. 5 mg. l-1 BAP 3% Sucrose 0. 5 mg. l-1 NAA 0. 1 mg. l-1 BAP 3% Sucrose 0. 5 mg. l-1 NAA 5. 0 mg. l-1 BAP
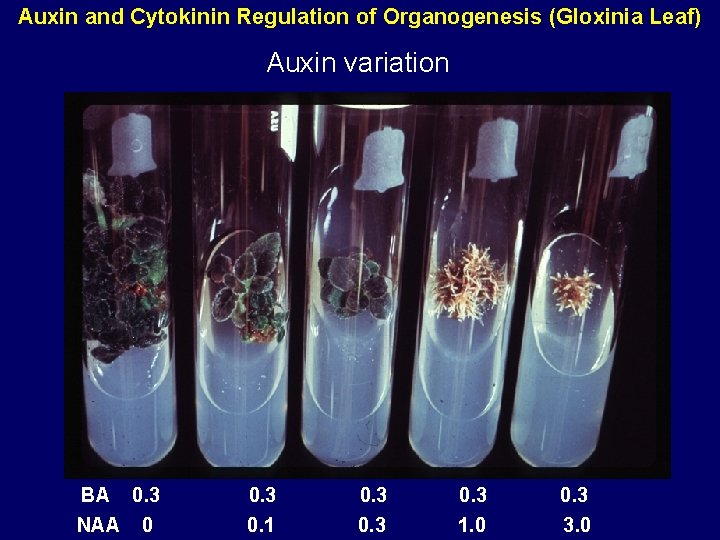
Auxin and Cytokinin Regulation of Organogenesis (Gloxinia Leaf) Auxin variation BA 0. 3 NAA 0 0. 3 0. 1 0. 3 1. 0 0. 3 3. 0
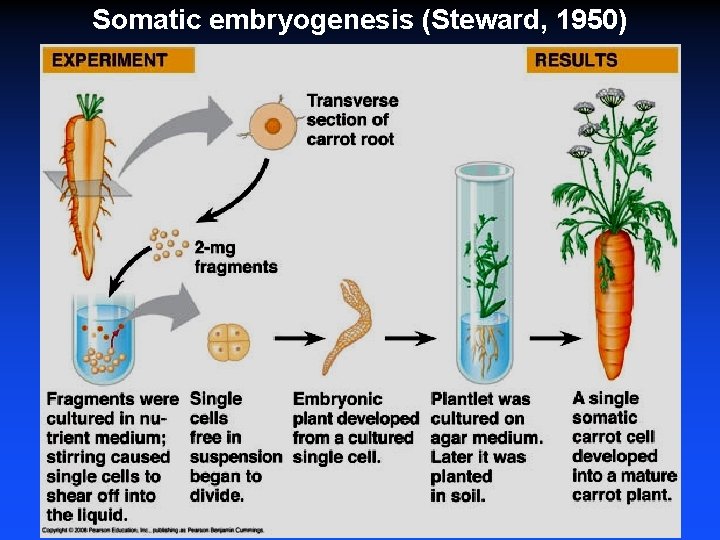
Somatic embryogenesis (Steward, 1950)
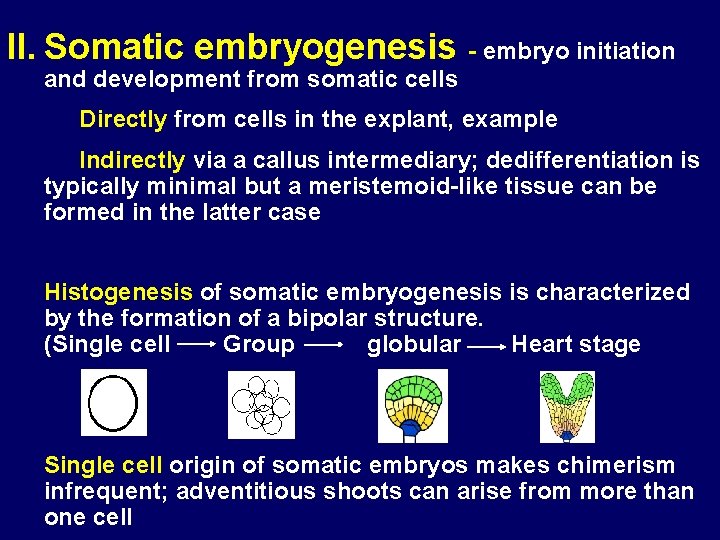
II. Somatic embryogenesis - embryo initiation and development from somatic cells Directly from cells in the explant, example Indirectly via a callus intermediary; dedifferentiation is typically minimal but a meristemoid-like tissue can be formed in the latter case Histogenesis of somatic embryogenesis is characterized by the formation of a bipolar structure. (Single cell Group globular Heart stage Single cell origin of somatic embryos makes chimerism infrequent; adventitious shoots can arise from more than one cell
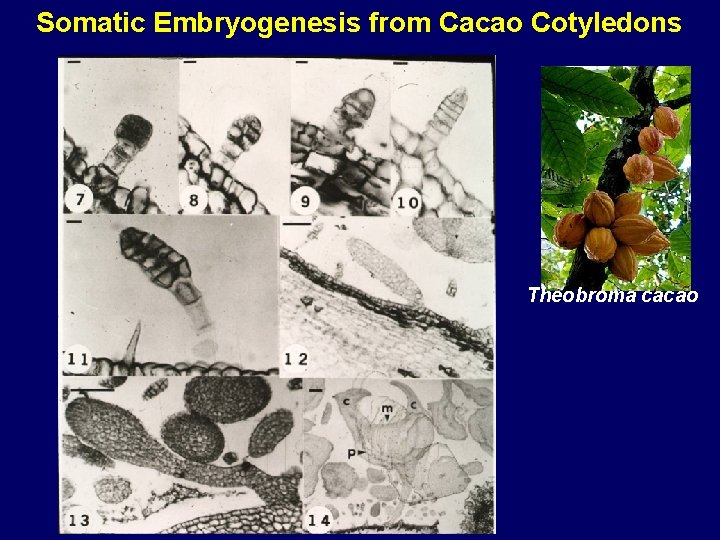
Somatic Embryogenesis from Cacao Cotyledons Theobroma cacao
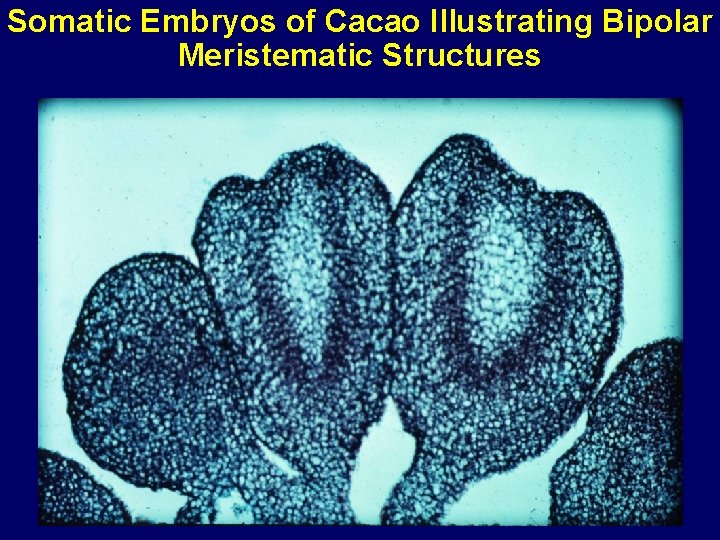
Somatic Embryos of Cacao Illustrating Bipolar Meristematic Structures

Acclimatization / Hardening off & field transfer In vivo: within a living organism Ex vivo: Outside a living organism In vitro: controlled environment outside of a living organism Ex vitro: Organisms removed from tissue culture and transplanted to soil or potting mixture In situ: In its original place Ex situ: off-site conservation of endangered species

Acclimatization & field transfer 3 peat : 1 vermiculite mixture Peat: decomposed vegetable matter Vermiculite/Perlite: hydrous, silicate mineral

Acclimatization & field transfer in vitro to ex vitro 3 peat : 1 vermiculite mixture High humidity

Acclimatization & field transfer

Acclimatization & field transfer Tissue Cultured Plants (culture induced phenotypes: CIP) have: 1. Less wax on leaf surfaces to inhibit water loss (extreme evapotranspiration) 2. Smaller and fewer palisade cells 3. larger mesophyll air space (air space in the middle of the leaf). 4. Stomata, the pores do not operate properly in tissue cultured plants (fresh leaves ex vitro) 5. Necessary to convert from a mixotrophic to a fully autotrophic mode of nutrition to survive (O 2 &/ sugar reduction)

Acclimatization & field transfer Acclimatization three types : 1. No Worry Methods removing plants from the test tubes or culture jars, placing them in growing media (such as peat: perlite). No moisture control. (good for herbaceous plants) 2. Mist Bench/Poly Tent Methods (+ cold treatment) (gradually lower humadity) 3. Humidification Chamber Methods (micron size droplets are emitted from a humidifier to the air surrounding the plants) (gradually lower humadity)

Acclimatization & field transfer in vitro to ex vitro

Acclimatization & field transfer
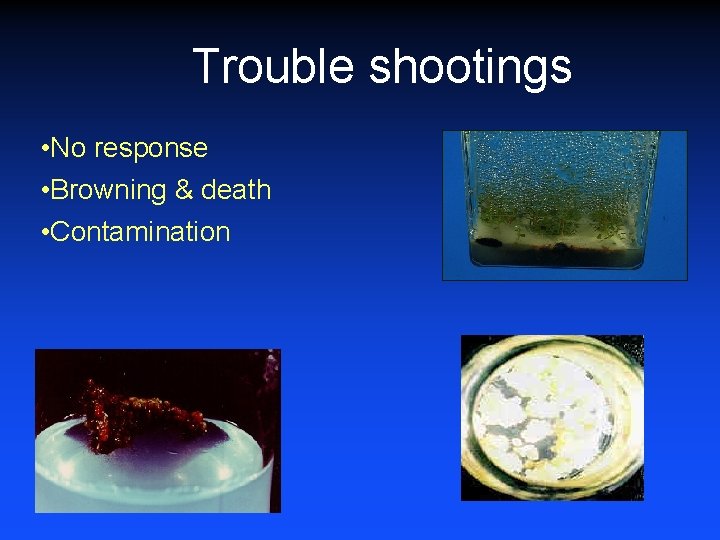
Trouble shootings • No response • Browning & death • Contamination

Trouble shootings No response: • explant &/ culture medium manipulation Browning & death: • Increased volume of medium • wax sealing of cut ends of explants • addition of charcoal in medium • addition of Pot. citrate & citrate (Anto. Ox) • Addition of polyvinylpyrrolidone (PVP) Contamination: • improve surface sterilization • add antibiotics in medium
- Slides: 59