Plant Protoplasts Plant Protoplasts n n Plant cells













- Slides: 23

Plant Protoplasts

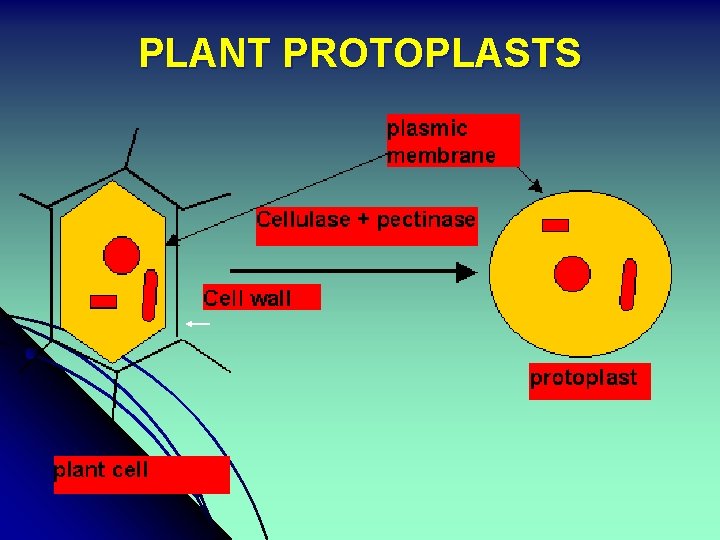
Plant Protoplasts n n Plant cells from which cell wall is removed They consist of the original cell contents bounded by the plasms membrane

PLANT PROTOPLASTS

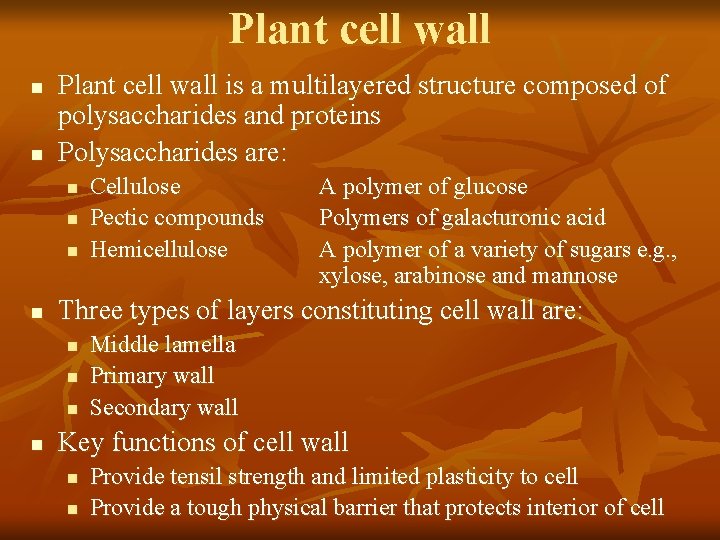
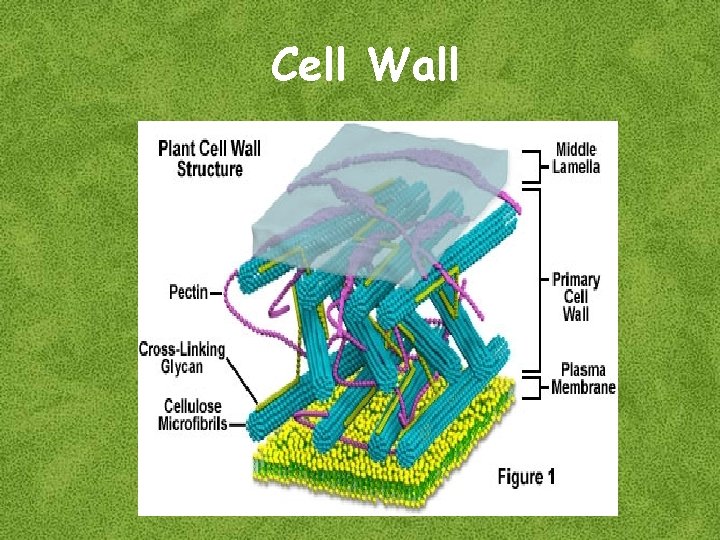
Plant cell wall n n Plant cell wall is a multilayered structure composed of polysaccharides and proteins Polysaccharides are: n n A polymer of glucose Polymers of galacturonic acid A polymer of a variety of sugars e. g. , xylose, arabinose and mannose Three types of layers constituting cell wall are: n n Cellulose Pectic compounds Hemicellulose Middle lamella Primary wall Secondary wall Key functions of cell wall n n Provide tensil strength and limited plasticity to cell Provide a tough physical barrier that protects interior of cell

Cell Wall

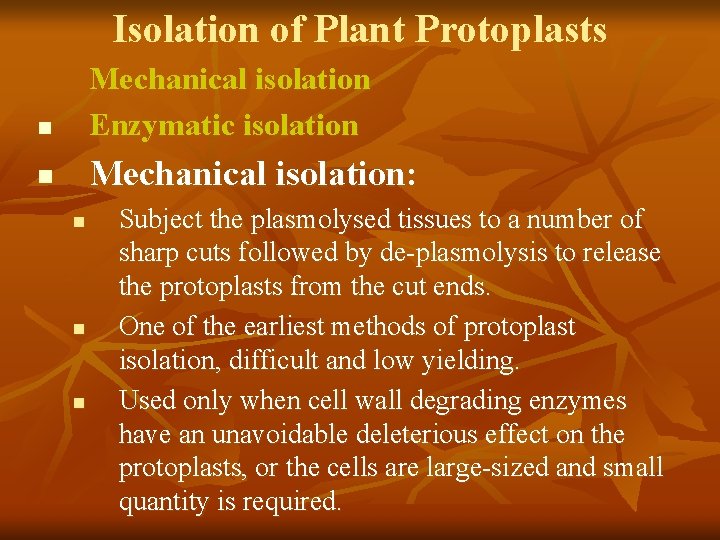
Isolation of Plant Protoplasts n Mechanical isolation Enzymatic isolation n Mechanical isolation: n n n Subject the plasmolysed tissues to a number of sharp cuts followed by de-plasmolysis to release the protoplasts from the cut ends. One of the earliest methods of protoplast isolation, difficult and low yielding. Used only when cell wall degrading enzymes have an unavoidable deleterious effect on the protoplasts, or the cells are large-sized and small quantity is required.


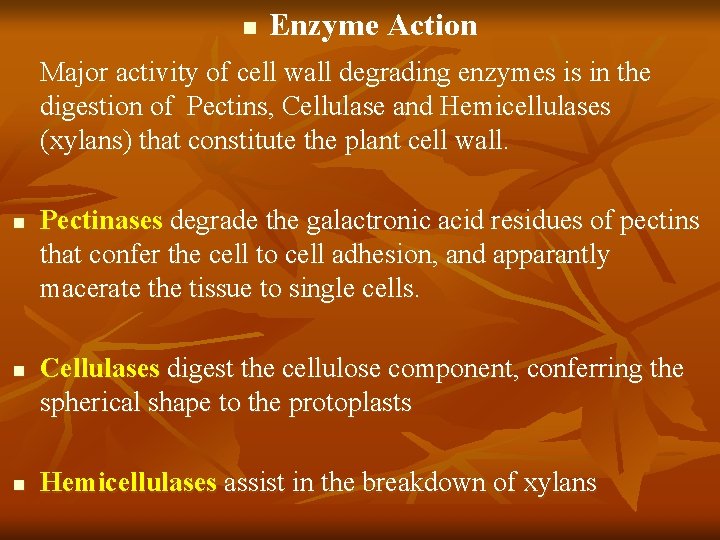
n Enzyme Action Major activity of cell wall degrading enzymes is in the digestion of Pectins, Cellulase and Hemicellulases (xylans) that constitute the plant cell wall. n n n Pectinases degrade the galactronic acid residues of pectins that confer the cell to cell adhesion, and apparantly macerate the tissue to single cells. Cellulases digest the cellulose component, conferring the spherical shape to the protoplasts Hemicellulases assist in the breakdown of xylans

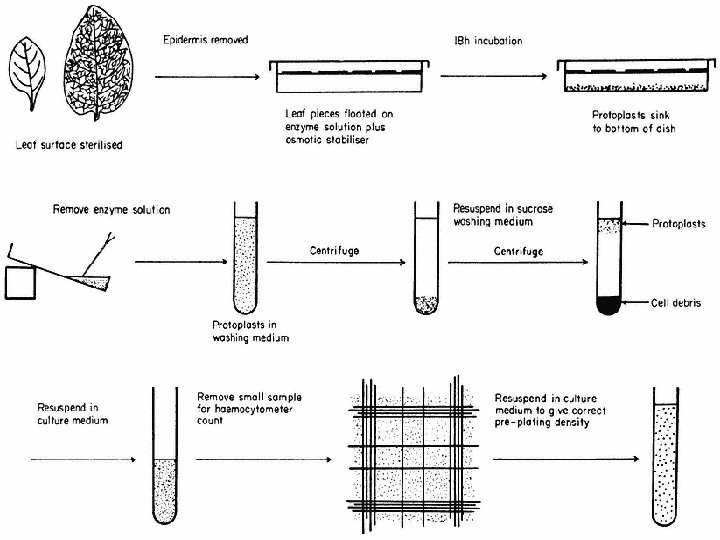
Protoplast isolation steps n n n n Selection of source material Sterilization of the tissue Preparation, filteration of enzymes Incubation of the tissue with the enzyme solution Release of protoplasts Filteration of protoplast solution through an autoclaved mesh to remove undigested tissues Repeated washing by centrifugation to remove enzymes Separation of protoplasts from debris Since removal of the cell wall results in loss of wall pressure upon the cell, protoplasts are isolated and maintained in hypertonic plasmolytica provided by a balanced inorganic salt medium or monosaccharide sugar solution. Mannitol, for example, is not readily transported across the plasmalemma and therefore provides a stable osmotic environment for the protoplast.

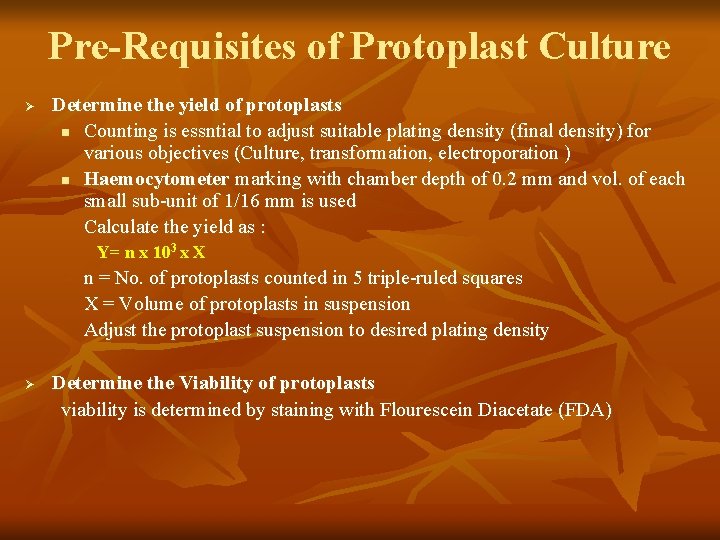
Pre-Requisites of Protoplast Culture Ø Determine the yield of protoplasts n Counting is essntial to adjust suitable plating density (final density) for various objectives (Culture, transformation, electroporation ) n Haemocytometer marking with chamber depth of 0. 2 mm and vol. of each small sub-unit of 1/16 mm is used Calculate the yield as : Y= n x 103 x X n = No. of protoplasts counted in 5 triple-ruled squares X = Volume of protoplasts in suspension Adjust the protoplast suspension to desired plating density Ø Determine the Viability of protoplasts viability is determined by staining with Flourescein Diacetate (FDA)

Protoplast Culture n Culture media: n n n n Most culture media are based on MS (Murashige & Skoog, 1962) and B 5 (Gamborg et al, 1968) with the addition of an osmoticum (nonmetabolizing sugar alcohol such as mannitol or somewhat soluble, sorbitol) Complex medium with coconut milk (Kao & Michayluk, 1975) used for the culture of protoplasts at very low densities Major growth regulators auxins and cytokinins are normally essential for sustained protoplast growth Some exceptions are requirement of only auxins e. g. , in carrot and A. thaliana. Auxins and cytokinins are detrimental to the growth of citrus protoplasts Growth requirement of protoplasts often change during culture, necessitating modification of medium composition, (reduction of the auxin conc). Sucrose and glucose are regular choices of carbon sources in most media, in cereals a change in carbon source from sucrose to maltose promotes regeneration. Agarose as solidifying agent gives better results than agar

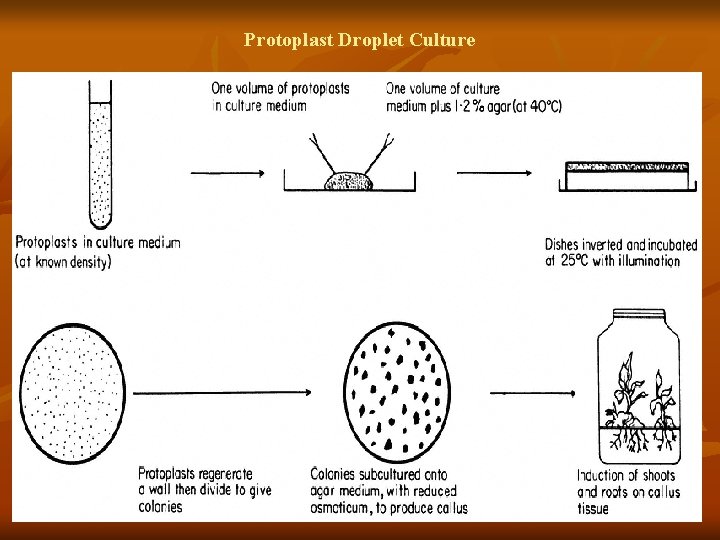
Protoplast culture methods 1) Liquid layers 2) Embedded in agarose/agar 3) Liquid over agarose 4) Alginate encapsulation 5) Hanging/sitting droplet culture 6) Filter paper substratum placed on agar 7) Microdrop array technique

Protoplast Droplet Culture

Maintenance of Protoplast Cultures n n n Incubate cultures for 2 wks in dark at 25 -30°C depending upon the crop species Afterwards transfer to continuous illumination from 1000 lux to 3000 lux. Cell wall formation is evident by the change of protoplast shape Division starts by 3 -7 d of culture initiation Sequential dilution of osmoticum of the culture medium Once callus formation is achieved plant regeneration process is the same as of regular tissue culture technique.

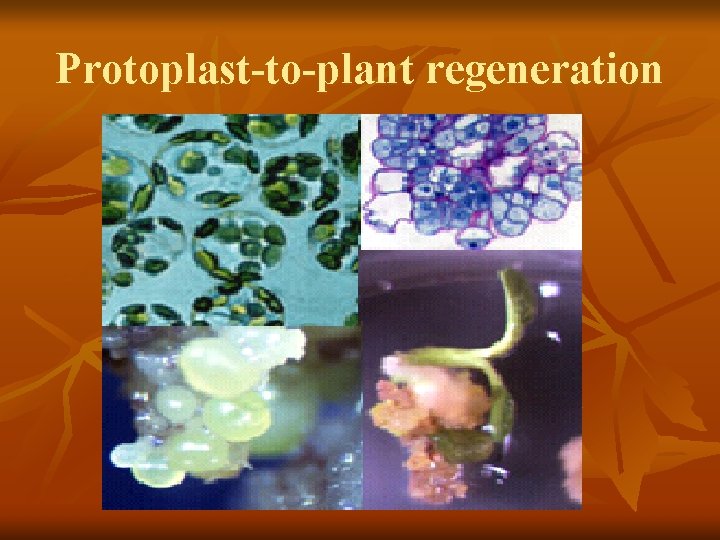
Protoplast-to-plant regeneration

Exploitation of Protoplast-to-plant Technologies n n Somatic hybridization to generate novel plants Transformation of protoplasts n DNA uptake into the nucleus of isolated protoplasts n Organelle transformation n Molecular Farming n Somaclonal variation

n n n Protoplasts may be produced, under aseptic conditions, from a wide range of plant species either directly from the whole plant, or indirectly from in vitro cultured tissues. From a physiological viewpoint, however, the protoplast cannot be regarded simply as a cell lacking a wall, since the mechanics of isolation, in conjunction with environmental factors, undoubtedly influence its metabolism and elicit subtle ultrastructural changes. The absence of a functional cell wall may affect the permeability of the cell membrane and lead to a general leakage of solutes from the protoplast. The protoplast is also in a transient state since most protoplasts, irrespective of their immediate cultural environment, will initiate the synthesis of a new cell wall a few hours after release, and eventually revert to a single-walled cell. In spite of these considerations, protoplasts provide an important biochemical tool for the biologist. In the absence of a cell wall, the exposed plasmalemma can be examined in great detail with respect to particle uptake, permeability, possible membraneassociated functions such as disease resistance, membrane fusion, and, during cell wall synthesis, the relationship of the plasmalemma to its cell wall.

Details of slide # 3 ed. Middle layer lamella first The of the wall and is shared by adjacent cells. It is composed of pectic compounds and proteins The primary wall: Formed after the middle lamella consisting of a framework of cellulose microfibrils embedded in a gel-like matrix of pectic compounds, hemicellulose and glycoproteins. Secondary wall is formed after cell anlargement, a rigid structure composed of cellulose, hemicellulase and lignin. Cell wall fulfills several important roles but two main fnctions are 1. To provide tensil strength and limited plasticity so that the cell can develop high turgor pressures without rupturing. Turger pressure provides support for nonwoody plants. 2. Provide a tough physical barrier tat protects the interior of cell from incvading microorganisms. The properties of plant cell wall that make it an efficient protective barrier restrict the use of plant cells for a variety of cell and tissue culture techniques, including the delivery of molecules into the cell, somatic hybridization and genetic manipulation. These techniques can only be applied to plant cells after removal of cell wall. n

When large populations of the protoplasts are required which is the norm, enzymatic digestion of cell wall is essential. Interestingly it was the release of protoplasts by natural degradation of cell wall during fruit ripening that stimulated investigations more than 4 decades ago of protoplast isolation from roots of tomato seedlings by E. C. COCKING (1960, University of Nottingham). Protoplast preparation was at first done in two steps: At first was the middle lamella dissolved by pectinases, then was the cell wall broken down by cellulase. The process is further simplified by a mixture of enzymes The enzymes required for this procedure are usually not pure, but crude extracts from certain bacteria and fungi. Nowadays is the method often shortened to just one operation in which a mixture of enzymes is applied.

Details of slide no. 10 Determine the yield of protoplasts Counting is essntial to adjust suitable protoplast plating density (final density) for various objectives (Culture, transformation, electroporation ). Plating density of protoplasts in the culture medium is crucial for maximising wall regeneration and concomitant daughter cell formation. Generally plating density is in the ranges 5 x 10 1 6 to 1 x 10 protoplasts per ml. An exessive high p. density rapidly depletes nutrients an protoplast-derived cells can fail to undergo sustained division Cells stimulate mitotic division of adjacent cells by releasing growth factors including amino acids into the surrounding medium, a process known as medium conditioningor nurse culture. Consequently, when protoplasts are cultured below the minimum inoculum density threshold they don’t survive as there is no medium conditioning. Haemocytometer marking with chamber depth of 0. 2 mm and vol. of each small sub-unit of 1/16 mm is used for counting protoplasts. i. Isolated protoplasts adjusted in final vol. of 10 ml of medium ii Moisten edges of haemocytometer and place cover slip firmly iii. Add a drop of well-mixed protoplast suspension to the counting chamb of H. cytom. iv. Count the no. of protoplasts in 5 triple-ruled squares each with 16 sigle ruled small squares v. Calculate the yield : Y= n x 103 x X n = No. of protoplasts counted in 5 triple-ruled squares X = Volume of protoplasts in suspension vi. Adjust the protoplast suspension to desired plating density

FDA is relatively nonpolar and passes freely across the plasma membrane. In association with viable protoplasts/cells, the molecules are hydrolyzed by the action of esterases to release polar fluorescein molecules which can not cross the membrane and consequently accumulate in the cell and stain it bright green under UV illumination. Thus, metabolically active protoplasts are visualized. Non-viable protoplasts appear orange red. Fluorescence Phase Contrast microscope is required.

Slide # 11 Isolated protoplasts commence cell wall regeneration within a short time (often minutes) following introduction into culture. However, they require osmotic protection until their primary walls can counteract the turgor pressure exerted by the cytoplasm. In some cases, gradual reduction of osmotic pressure by diluting the culture medium with a solution of similar composition but of reduced osmotic pressure is essential for sustaining mitotic division, leading to formation of daughter cells and tissues. Protoplasts from different species and from different tissues of the same species may vary in their nutritional requirements. n Many culture media have been based on MS (Murashige & Skoog, 1962) and B 5 (Gamborg et al, 1968) formulations with the addition of an osmoticum, usually a nonmetabolisable sugar alcohol such as mannitolor somewhat soluble, sorbitol. Ideally media should be simple and fully defined to ensure reproducibility b/w labs. n An exception is the complex, undefined medium containing coconut milk (Kao & Michayluk, 1975) used for the culture of protoplasts at very low densities. Major growth regulators auxins and cytokinins are normally essential for sustained protoplast growth , some exceptions are requirement of only auxins e. g. , in carrot and A. thaliana. Auxins and cytokinins are detrimental to the growth of citrus Growth requirement of protoplasts often change during culture, necessitating modification of Medium composition, typically involving reduction of the auxin conc. Sucrose and glucose are regular choices of carbon sources in most media, in cereals a change in carbon source from sucrose to maltose promoted regeneration. n Several approaches are developed for protoplast culture all of which are based on liquid or semi-solid media, or their combination Protoplasts are plated by one of the methods: 1)Liquid layers. 2) Embedded in agarose/agar. 3) Liquid over agarose 4) Alginate encapsulation 5) Hanging/sitting droplet culture. 6) Filter paper substratum placed on agar. 7) Microdrop array technique Agarose has been used in place of gar Maintenance of protoplast culture

# 15. Protoplast-to-plant technologies are being exploited in different areas of plant cell research. Somatic hybridization to generate novel plants: A no. of recent papers have describes the generation of unique plants through somatic hybridization by protoplast fusion. Citrus, Brassica, Potato and other members of Solanaceae, cereals, ornamentals and miscellaneous crop plants have been targetted for the incorporation of useful traits via this technique. n n Transformation of protoplasts: n DNA uptake into the nucleus of isolated protoplasts Because the plasma membrane has fluid mosaic characteristics, DNA uptake can be induced by chemical /or physical procedures. Treatment of protoplast-plasmid mixtures with PEG or Electroporation is the approach normally exploited to induce DNA uptake into protoplasts. However, transformation frequencies remain low, owing to the reproducible protoplast-to-plant system. PPT can be cotransformed with more than one gene carried on the same or separate plasmids. This strategfy is especially important in transforming plants that are not amenable to other methods of gene delivery. n Organelle transformation The advantage of plastid transformation over nuclear transformation include high foreign protein synthesis in these organelles and the absence of epigenetic effects in regenerated plants. IOne key attraction of chloroplast engineering is the apparent lack of transgene transmission through pollen. The recent success in plastid transformation gives hopes of successful future transformation of mitochondria as well. Molecular Farming: It years there has been growing interest in the use of plants as expression systems for the production of recombinant proteins. At present several plant-derived pharmaceutical proteins are at an advanced stage of commercial development, such as antibiotics, vaccines, human blood products, hormones and growth regulators Somaclonal hybridization variation somatic approaches for combining interspecific and intergeneric traits and targeted modification of genomes, however, somaclonal variation or protoclonal variation may involve expression and release of naturally occuring genetic variation as a result of culture and may be regarded as a simple form of genetic manipulation. Protoclonal variation is more evident in the form of alterations in morphological characters of the plants. The attraction os somaclonal or protoclonal variation is that it requires no knowledge of the genetic basis of specific traits, it negates DNA recombinant techniques, it does not require mutagenic agents, specialized apparatus and it can be exploited through routine culture procedures.
 Animal cell plant cell venn diagram
Animal cell plant cell venn diagram Chlorocruorin
Chlorocruorin Masses of cells form and steal nutrients from healthy cells
Masses of cells form and steal nutrients from healthy cells Thyroid parafollicular cells
Thyroid parafollicular cells Cell substance
Cell substance Nondisjunction in meiosis
Nondisjunction in meiosis What is eukarya
What is eukarya Younger cells cuboidal older cells flattened
Younger cells cuboidal older cells flattened Somatic cells vs gametes
Somatic cells vs gametes Prokaryotic vs eukaryotic cells venn diagram
Prokaryotic vs eukaryotic cells venn diagram Onodi cells
Onodi cells Cuál es la diferencia entre la célula animal y vegetal
Cuál es la diferencia entre la célula animal y vegetal Why dna is more stable than rna
Why dna is more stable than rna Organelle trail
Organelle trail Alpha intercalated cell
Alpha intercalated cell Prokaryotic cells
Prokaryotic cells Whats the difference between plant and animal cells
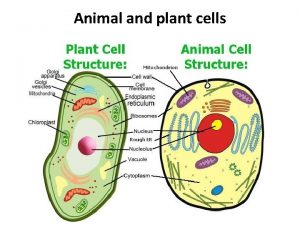
Whats the difference between plant and animal cells Animal vs plant cell
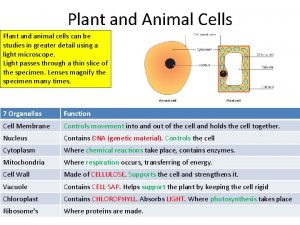
Animal vs plant cell Plant and animal cells
Plant and animal cells Microtubules in plant cells
Microtubules in plant cells Plant sex cells
Plant sex cells Perixomes
Perixomes The incredible edible cell
The incredible edible cell Animal cell and plant cell
Animal cell and plant cell