Plant Microtechnique BOT 213 Course Objectives Managing the

Plant Microtechnique BOT 213 Course Objectives: Managing the techniques of microscopic slides making, microscopic measurements and methods of identification of some organic compounds in plant cells. Course Outcomes: After finishing this course, students should be able to: - Make temporary microscopic slides, using different cutting techniques and permanent microscopic slides using paraffin method. - Doing microscopic measurements using image analyzing programs - Detect the presence of different groups of organic compounds in plant material

Course Content: § Preparation of plant material for microscopic slides. § Types of microscopic slides. Methods of sections. § Types of microtomes and principles of their work. Methods of temporary and permanent microscopic slides. § Temporary slides. § Permanent slides – paraffin method. § Special methods (maceration and squash methods). Microscopic measurements. § Methods of microscopic measurement and data processing (standard and stereological method; measurements using Image Analyzing System and light microscope). § Basic histological methods.
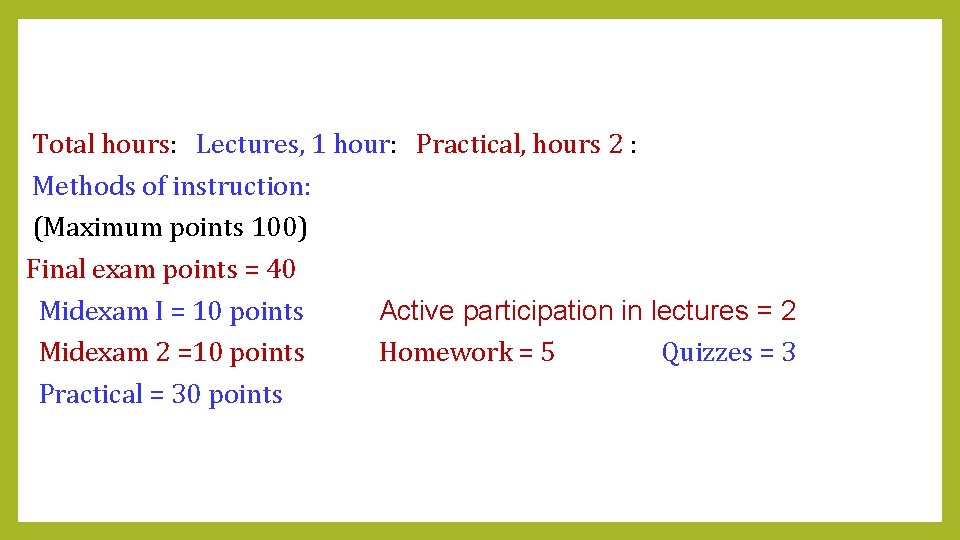
Total hours: Lectures, 1 hour: Practical, hours 2 : Methods of instruction: (Maximum points 100) Final exam points = 40 Midexam I = 10 points Active participation in lectures = 2 Midexam 2 =10 points Homework = 5 Quizzes = 3 Practical = 30 points

References: 1. Jensen, W. A. (1962): Botanical Histochemistry. W. H. Freeman and Company, USA. 2. Ruzin, S. (1999): Plant Microtechnique and Microscopy. Oxford University Press Inc. , Oxford. 3 Plant Microtechniques and Protocols Editors: Yeung, E. C. T. , Stasolla, C. , Sumner, M. J. , Huang, B. Q. (Eds. ) (2015) 4 - P L A N T M I C R O T E C H N I Q U E BY DONALD ALEXANDER JOHANSEN 1 FIRST E D I T I ON T H I R D I M P R E S S I ON Mc. GRAW- H I L L BOOK COMPANY, INC. N E W Y O R K A N D L O N D O N 1940


Sass ( 1958) was defined it as the science consists of three overlapping activities with each other: (1) Sample Preparation for microscopic study. (2) the proper use of the microscope and related devices help to explain the study samples. (3) codification of results and drawing which was replaced in modern imaging cameras , because of its major role in the transfer of the real image of the sample, so no interference from the researcher or the examiner in few modifications. And for the development and diversity of this science, it was divided into three sections: : ﻫﻲ ﺍﻟﺒﻌﺾ ﺑﻌﻀﻬﺎ ﻣﻊ ﻣﺘﺪﺍﺧﻠﺔ ﻧﺸﺎﻃﺎﺕ ﺛﻼﺙ ﻣﻦ ﻳﺘﻜﻮﻥ ﻋﻠﻢ ﻫﻮ : ﺑﻘﻮﻟﻪ ﻡ 1958 ﻋﺎﻡ Sass ﺳﺎﺱ ﻋﺮﻓﻪ ﻭﻗﺪ . ﺍﻟﻤﺠﻬﺮﻳﺔ ﻟﻠﺪﺭﺍﺳﺔ ﺍﻟﻌﻴﻨﺔ ( ﺗﺤﻀﻴﺮ 1 ) . ﺍﻟﻌﻴﻨﺔ ﻭﺩﺭﺍﺳﺔ ﻟﺘﻔﺴﻴﺮ ﻣﺴﺎﻋﺪﺓ ﺃﺠﻬﺰﺓ ﻣﻦ ﺑﻪ ﻳﺘﻌﻠﻖ ﻭﻣﺎ ﻟﻠﻤﺠﻬﺮ ﺍﻟﺼﺤﻴﺢ ( ﺍﻻﺳﺘﻌﻤﺎﻝ 2 ) ﺃﻮ ﺍﻟﺒﺎﺣﺚ ﻳﺪ ﻓﻴﻬﺎ ﺗﺘﺪﺧﻞ ﻻ ﺍﻟﺘﻲ ﺍﻟﺤﻘﻴﻘﻴﺔ ﺍﻟﻌﻴﻨﺔ ﺻﻮﺭﺓ ﻧﻘﻞ ﻓﻲ ﻛﺒﻴﺮ ﺩﻭﺭ ﻣﻦ ﻟﻪ ﻟﻤﺎ ﻟﻠﺮﺳﻢ ﺑﺪﻳﻼ ﺍﻷﺤﻴﺎﻥ ﺃﻐﻠﺐ ﻓﻲ ﺍﻟﺘﺼﻮﻳﺮ ﻳﺤﻞ ﻭﻗﺪ ﺍﻟﻨﺘﺎﺋﺞ ﻭﺭﺳﻢ ( ﺗﺪﻭﻳﻦ 3 ). ﺍﻟﻔﺎﺣﺺ

Quiz: Defined plant Microtechnique? Answer: It is the science consists of three overlapping activities with each other: (1) Sample Preparation for microscopic study. (2) the proper use of the microscope and related devices help to explain the study samples. (3) codification of results and drawing which was replaced in modern imaging cameras , because of its major role in the








Quiz: What are the Preparation types of large plant Material? Answer: 1 - Dry preparation 2 - Wet preparation

Quiz: What are the solutions used to retain the natural colour of the wet Plant sample? Answer: 1 - 20% glacial acetic acid solution 2 - Copper acetate

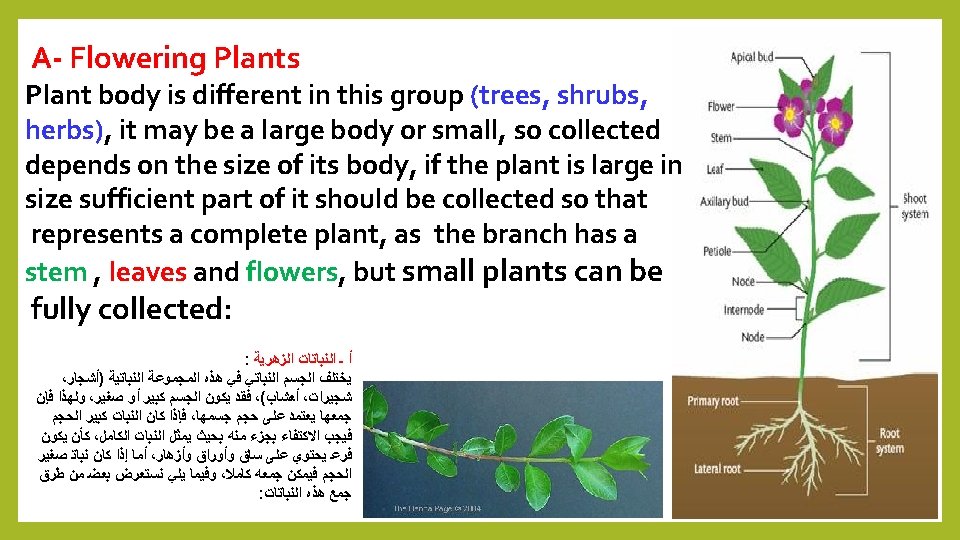




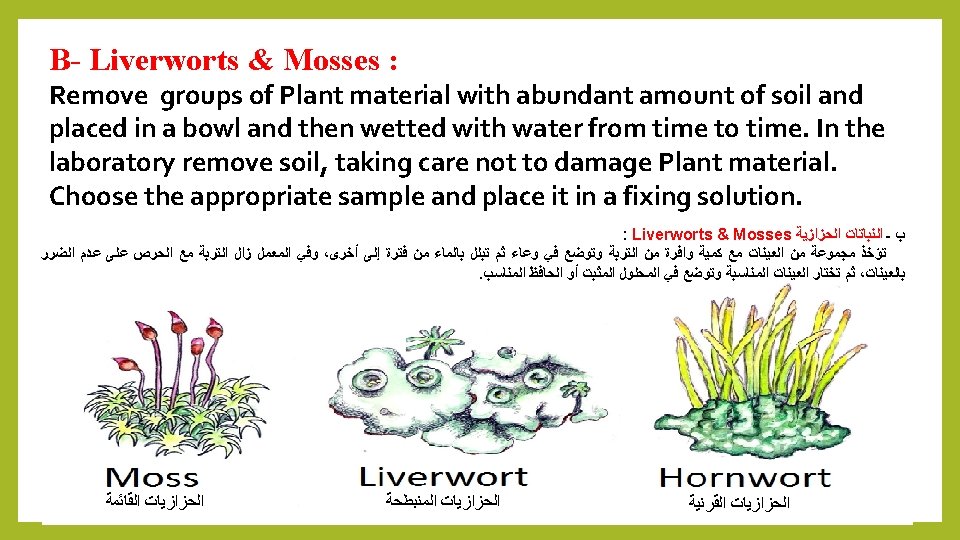




Quiz: Mention the types of the plant material to be prepared to examined under Light Microscope? Answer A- Flowering Plants( Root, stem. Leaves and Floral parts) B- Liverworts & Mosses. C- Algae. D- Pathological materials

Types of sample preparation: Sample preparation depends on the need of examination required , were divided into three types: 1 - Temporary preparation 2 - Semitemporary preparation 3 - Permanent preparation : ﺍﻟﻌﻴﻨﺎﺕ ﺗﺤﻀﻴﺮ ﺃﻨﻮﺍﻉ : ﻫﻲ ﺃﻨﻮﺍﻉ ﺛﻼﺛﺔ ﺇﻟﻰ ﺍﻟﻌﻴﻨﺎﺕ ﺗﺤﻀﻴﺮ ﺗﻘﺴﻴﻢ ﻭﻳﻤﻜﻦ ، ﻟﻬﺎ ﺍﺳﺘﻌﻤﺎﻟﻪ ﻭﻣﺪﻯ ﺍﻟﻔﺎﺣﺺ ﺣﺎﺟﺔ ﻋﻠﻰ ﺍﻟﻌﻴﻨﺔ ﺗﺤﻀﻴﺮ ﻳﻌﺘﻤﺪ Temporary preparation ﺍﻟﻤﺆﻘﺖ ﺍﻟﺘﺤﻀﻴﺮ ـ 1 Semitemporary preparation ﺍﻟﻤﺆﻘﺖ ﻧﺼﻒ ﺍﻟﺘﺤﻀﻴﺮ ـ 2 Permanent preparation : ﺍﻟﺘﺤﻀﻴﺮﺍﻟﻤﺴﺘﺪﻳﻢ ـ 3

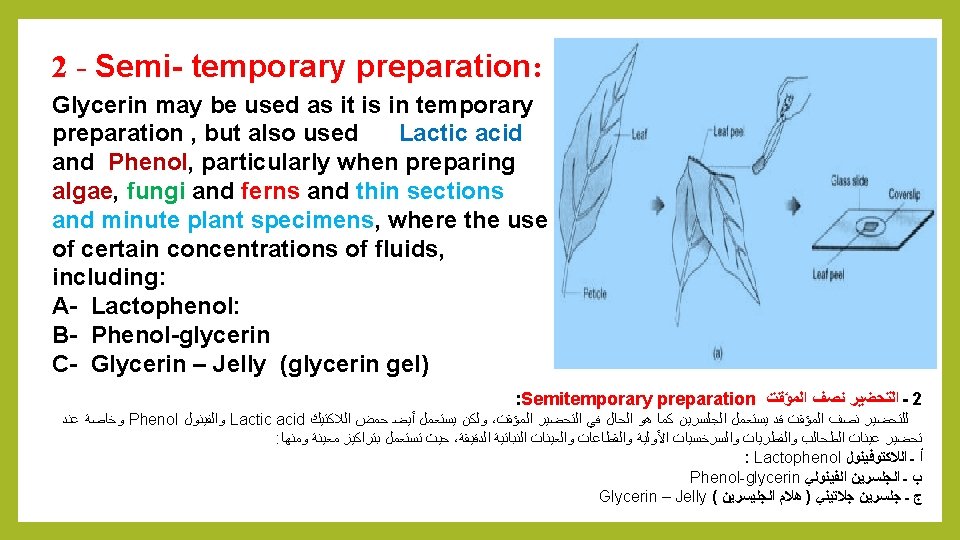


Quiz: What are the types of sample preparation? Answer: 1 - Temporary preparation 2 - Semitemporary preparation 3 - Permanent preparation:
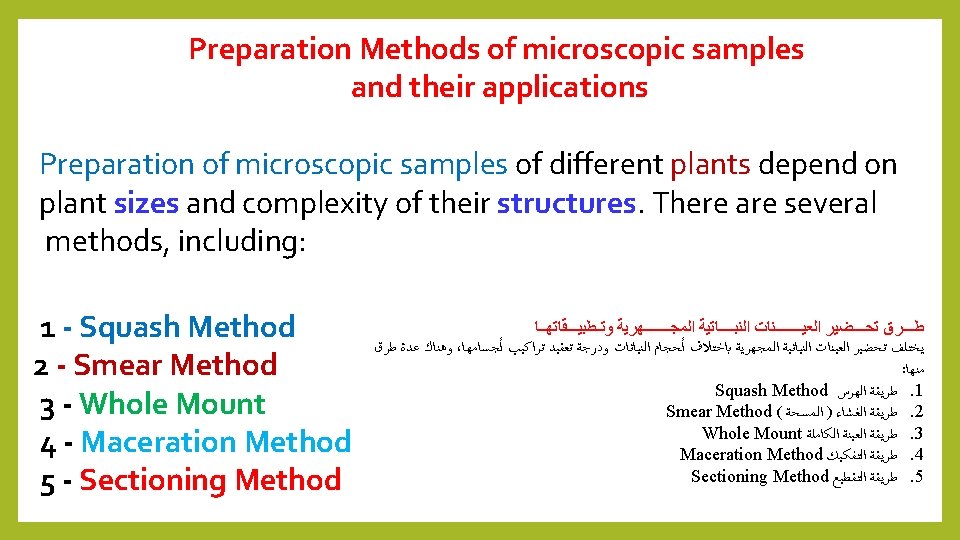

Quiz: Mention the types of Preparation Methods of microscopic samples? Answer: 1 - Squash Method 2 - Smear Method 3 - Whole Mount 4 - Maceration Method 5 - Sectioning Method



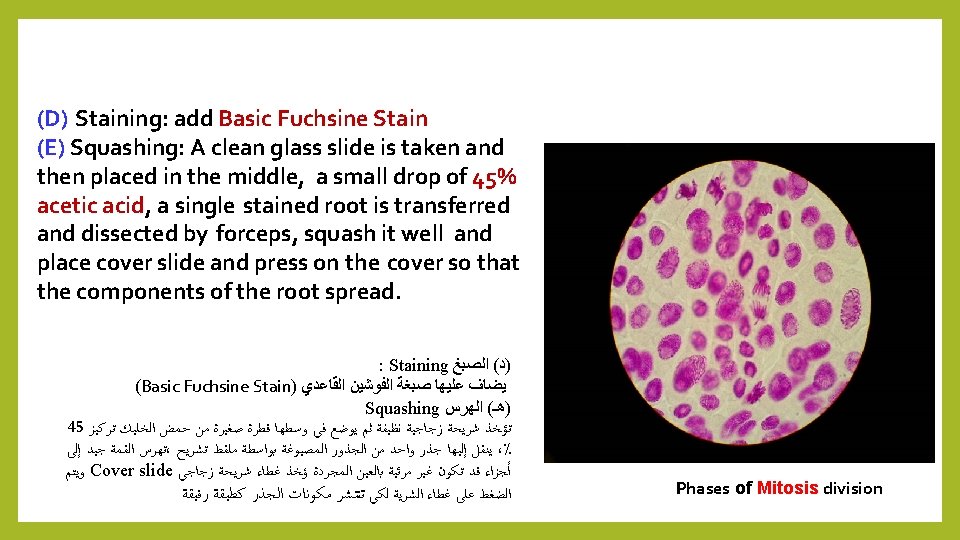
1 - Feulgen Method: 1 - Feulgen Method was discovered by Feulgen in 1924, a technical study of chromosomes or DNA and summarize in the following steps: (A) Root Germination (onion roots) (B) Killing and Fixation: by Carnoy's fluid. (C) Hydrolysis: the transfer of the roots to the test tube containing hydrochloric acid N HCl. Then placed in an oven or a water bath at 60 °C for 12 minutes. DNA ﺍﻭ ﺍﻟﺼﺒﻐﻴﺎﺕ ﻟﺪﺭﺍﺳﺔ ﺗﻘﻨﻴﺔ ﻭﻫﻲ 1924 ﻓﻮﻟﺠﻦ ﺍﻛﺘﺸﻔﻬﺎ Feulgen Method ﻓﻮﻟﺠﻴﻦ ﻃﺮﻳﻘﺔ ـ 1 : ﺍﻟﺘﺎﻟﻴﺔ ﺑﺎﻟﺨﻄﻮﺍﺕ ﻭﺗﺘﻠﺨﺺ Root Germination : ﺍﻟﺠﺬﻭﺭ ﺇﻧﺒﺎﺕ ( ﺃ ) Carnoy's fixative, : Killing and Fixation : ﻭﺍﻟﺘﺜﺒﻴﺖ ﺍﻟﻘﺘﻞ ( )ﺏ : Hydrolysis ﺍﻟﺘﺤﻠﻴﻞ ( )ﺝ N HCl ﻋﻴﺎﺭﻱ ﺍﻟﻬﻴﺪﺭﻭﻛﻠﻮﺭﻳﻚ ﺣﻤﺾ ﻋﻠﻰ ﺗﺤﺘﻮﻱ ﺍﺧﺘﺒﺎﺭ ﺃﻨﺒﻮﺑﺔ ﺇﻟﻰ ﺍﻟﺠﺬﻭﺭ ﺗﻨﻘﻞ . ﺩﻗﻴﻘﺔ 12 ﻟﻤﺪﺓ ﻡ 5 60 ﺩﺭﺟﺔ ﻋﻠﻰ ﻣﺎﺋﻲ ﺣﻤﺎﻡ ﺃﻮ ﻓﺮﻥ ﻓﻲ ﺗﻮﺿﻊ ﺛﻢ
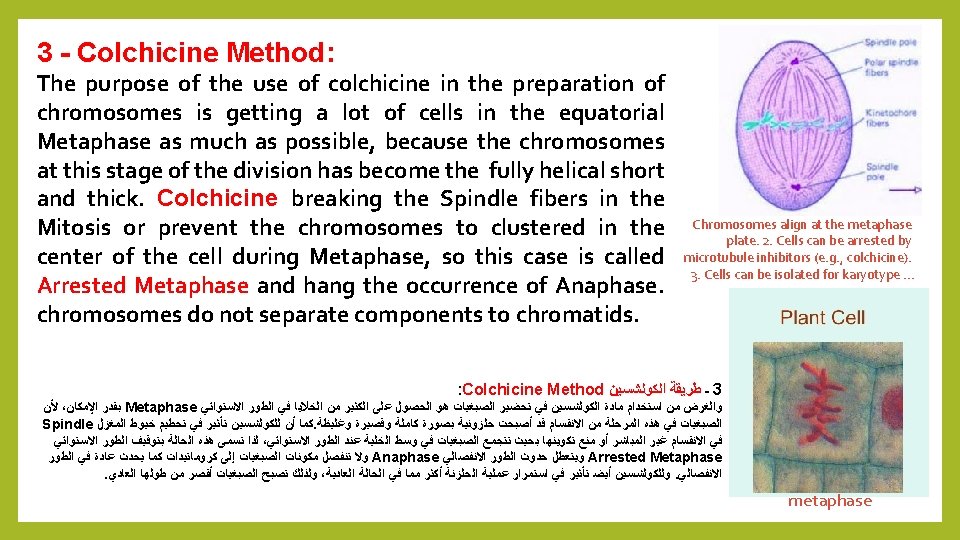
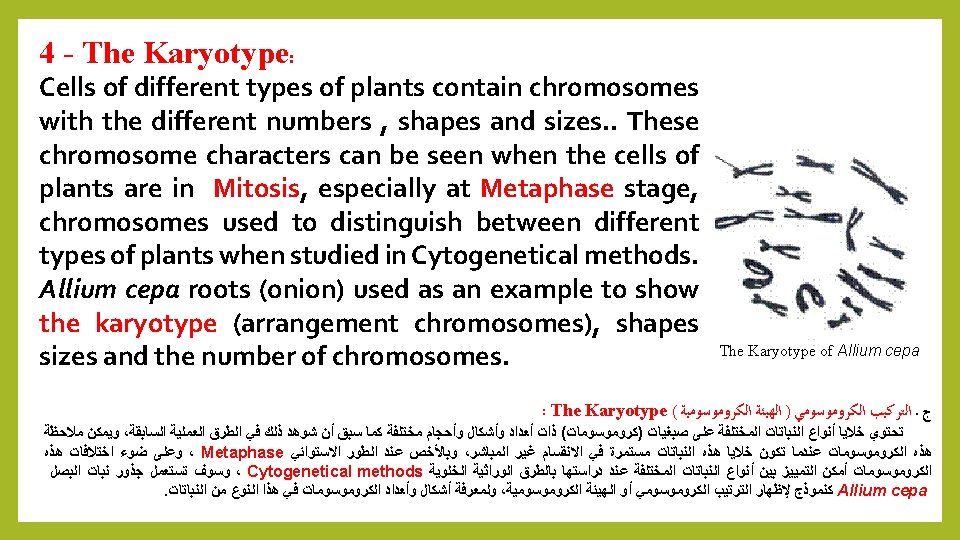

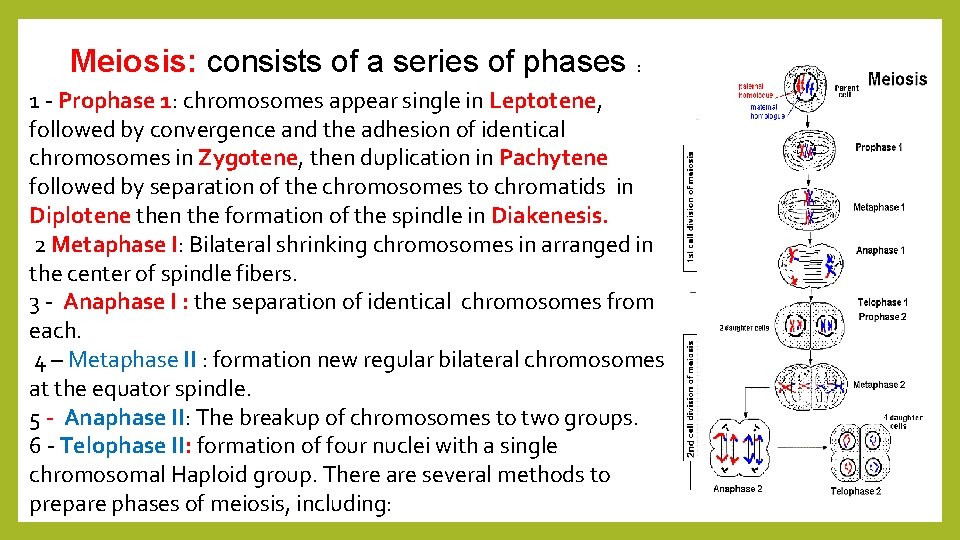
Meiosis: consists of a series of phases : 1 - Prophase 1: chromosomes appear single in Leptotene, followed by convergence and the adhesion of identical chromosomes in Zygotene, then duplication in Pachytene followed by separation of the chromosomes to chromatids in Diplotene then the formation of the spindle in Diakenesis. 2 Metaphase I: Bilateral shrinking chromosomes in arranged in the center of spindle fibers. 3 - Anaphase I : the separation of identical chromosomes from each. 4 – Metaphase II : formation new regular bilateral chromosomes at the equator spindle. 5 - Anaphase II: The breakup of chromosomes to two groups. 6 - Telophase II: formation of four nuclei with a single chromosomal Haploid group. There are several methods to prepare phases of meiosis, including:
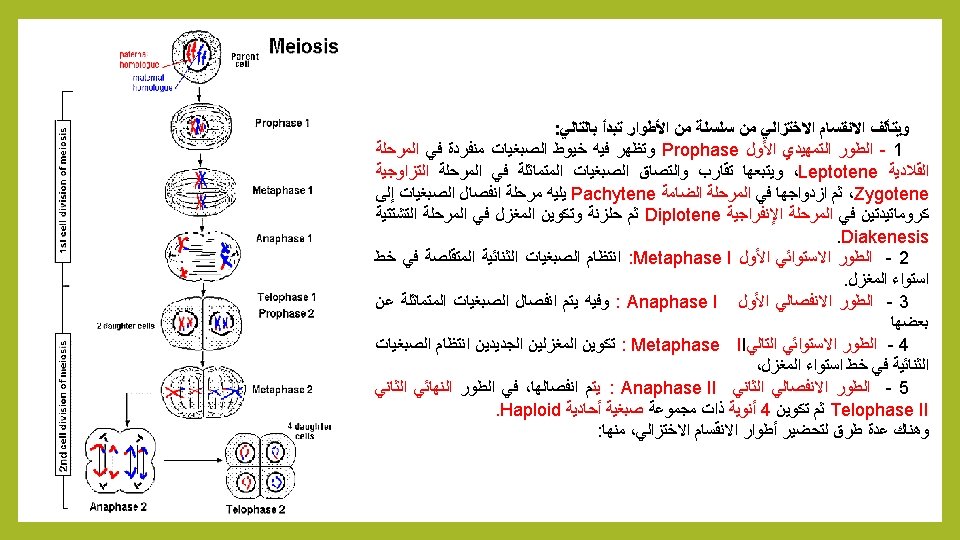

A – General Method 1 - Collect floral buds of plants to be studied. 2 - Squash in a drop of the selected stain, and then examine under a Light Microscope using a low-power (x 10) to see Anthers contain different stages of Meiosis, 3 - If desired phases clear. placed the cover slide. Gently heat with Bunsen Burner (avoid boiling dye), and then placed slide between two filter paper and press gently on the slide cover, and examine different stages of Meiosis, under a Microscope (High-power (x 40). 4 – Place Wax around the edges of the cover slide for temporary mounting.
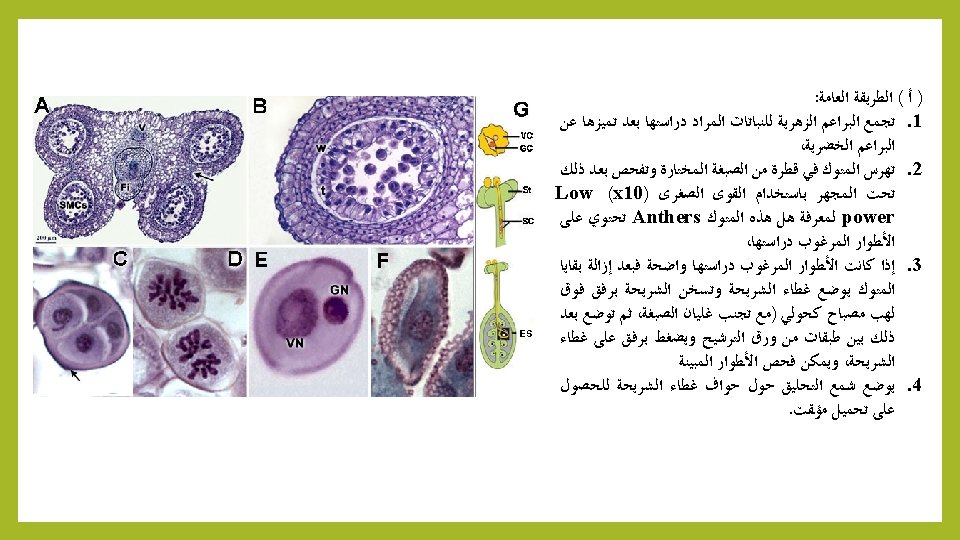

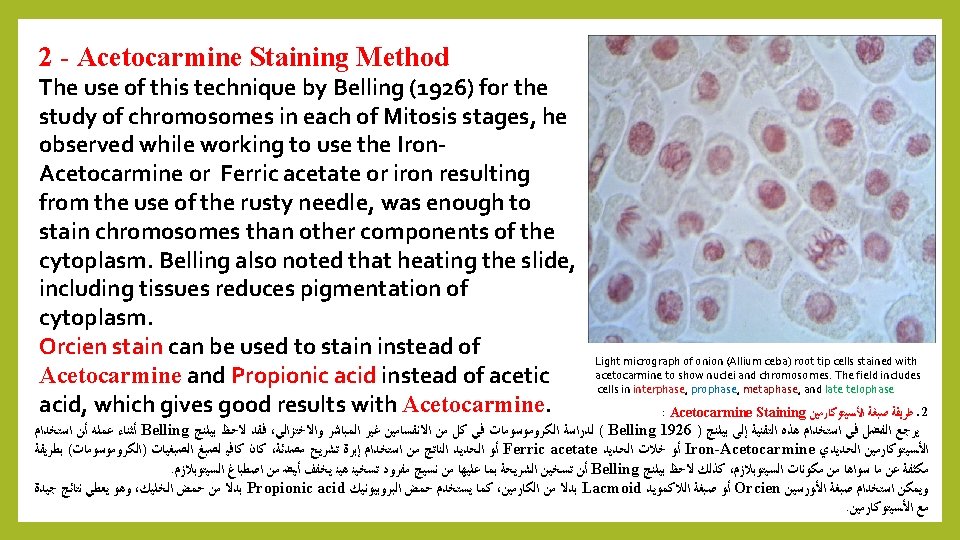
B – Belling Method: We have studied previously Belling method proposed by Belling in 1926 and it still used for the study of chromosomes during Meiosis in preparation of pollen grains using Iron-Acetocarmine dye (see practical). JOHN BELLING, "THE IRON-ACETOCARMINE METHOD OF FIXING AND STAINING CHROMOSOMES, " The Biological Bulletin 50, no. 2 (February 1926): 160 -162. DOI: 10. 2307/1536680 : Belling ﺑﻠﻨﺞ ) ﺏ ( ﻃﺮﻳﻘﺔ ( ﺍﻷﻤﻴﺔ ) ﺍﻟﻤﻮﻟﺪﺓ ﻟﻠﺨﻼﻳﺎ ﺍﻻﺧﺘﺰﺍﻟﻲ ﺍﻻﻧﻘﺴﺎﻡ ﺃﺜﻨﺎﺀ ﺍﻟﻜﺮﻭﻣﻮﺳﻮﻣﺎﺕ ﻟﺪﺭﺍﺳﺔ ﺍﻵﻦ ﺣﺘﻰ ﺗﺴﺘﺨﺪﻡ ﻣﺎﺯﺍﻟﺖ ﺃﻨﻬﺎ ﺇﻻ ﻡ 1926 ﻋﺎﻡ ﺑﻠﻨﺞ ﺍﻗﺘﺮﺣﻬﺎ ﺍﻟﺘﻲ ﺍﻟﻄﺮﻳﻘﺔ ﻫﺬﻩ ﻗﺪﻡ ﻣﻦ ﺑﺎﻟﺮﻏﻢ : ﺍﻟﺘﺎﻟﻴﺔ ﺑﺎﻟﺨﻄﻮﺍﺕ ﺗﻠﺨﻴﺼﻬﺎ ﻭﻳﻤﻜﻦ Iron- Acetocarmine ﺍﻟﺤﺪﻳﺪﻱ ﺍﻟﺨﻠﻲ ﺍﻟﻜﺎﺭﻣﻴﻦ ﺻﺒﻐﺔ ﺑﺎﺳﺘﺨﺪﺍﻡ ﺍﻟﻠﻘﺎﺡ ﻟﺤﺒﻮﺏ
c- Preparation of Pollen Grains : There are three methods followed for the preparation of pollen grains depending on the quality of microscopic examination: 1 – Preparation of pollen grains, examined under a Light Microscope. 2 - Preparation of pollen grains, examined under a scanning Electron Microscope. 3 - Preparation of pollen grains, examined under the Transmission Electron Microscope. : Preparation of Pollen Grains ﺍﻟﻠﻘﺎﺡ ﺣﺒﻮﺏ ﺗﺤﻀﻴﺮ ـ ﺝ : ﻭﻫﻲ ، ﺍﻟﻤﺠﻬﺮﻱ ﺍﻟﻔﺤﺺ ﻟﻨﻮﻋﻴﺔ ﺗﺒﻌ ﺍﻟﻠﻘﺎﺡ ﺣﺒﻮﺏ ﻟﺘﺤﻀﻴﺮ ﻣﺘﺒﻌﺔ ﻃﺮﻕ ﺛﻼﺙ ﻫﻨﺎﻙ . ﺍﻟﻀﻮﺋﻲ ﺍﻟﻤﺠﻬﺮ ﺗﺤﺖ ﻟﻠﻔﺤﺺ ﺍﻟﻠﻘﺎﺡ ﺣﺒﻮﺏ ﺗﺤﻀﻴﺮ. 1. ﺍﻟﻤﺎﺳﺢ ﺍﻟﻤﺠﻬﺮ ﺗﺤﺖ ﻟﻠﻔﺤﺺ ﺍﻟﻠﻘﺎﺡ ﺣﺒﻮﺏ ﺗﺤﻀﻴﺮ. 2. ﺍﻟﻨﺎﻓﺬ ﺍﻟﻤﺠﻬﺮ ﺗﺤﺖ ﻟﻠﻔﺤﺺ ﺍﻟﻠﻘﺎﺡ ﺣﺒﻮﺏ ﺗﺤﻀﻴﺮ. 3
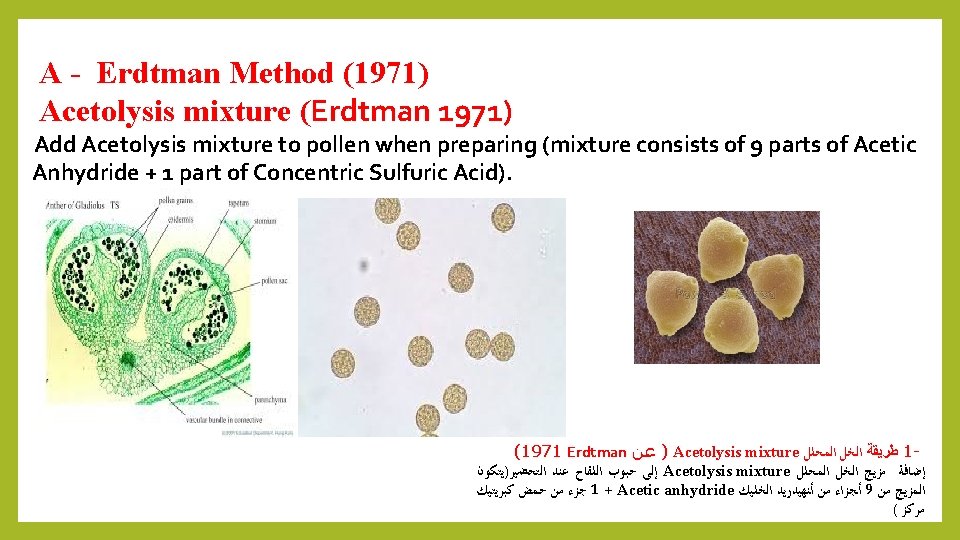
A - Erdtman Method (1971) Acetolysis mixture (Erdtman 1971) Add Acetolysis mixture to pollen when preparing (mixture consists of 9 parts of Acetic Anhydride + 1 part of Concentric Sulfuric Acid). (1971 Erdtman ) ﻋﻦ Acetolysis mixture ﺍﻟﻤﺤﻠﻞ ﻃﺮﻳﻘﺔ ﺍﻟﺨﻞ 1 ﺍﻟﺘﺤﻀﻴﺮ)ﻳﺘﻜﻮﻥ ﻋﻨﺪ ﺍﻟﻠﻘﺎﺡ ﺣﺒﻮﺏ ﺇﻟﻰ Acetolysis mixture ﺍﻟﻤﺤﻠﻞ ﺍﻟﺨﻞ ﻣﺰﻳﺞ ﺇﺿﺎﻓﺔ ﻛﺒﺮﻳﺘﻴﻚ ﺣﻤﺾ ﻣﻦ ﺟﺰﺀ 1 + Acetic anhydride ﺍﻟﺨﻠﻴﻚ ﺃﻨﻬﻴﺪﺭﻳﺪ ﻣﻦ ﺃﺠﺰﺍﺀ 9 ﻣﻦ ﺍﻟﻤﺰﻳﺞ ( ﻣﺮﻛﺰ

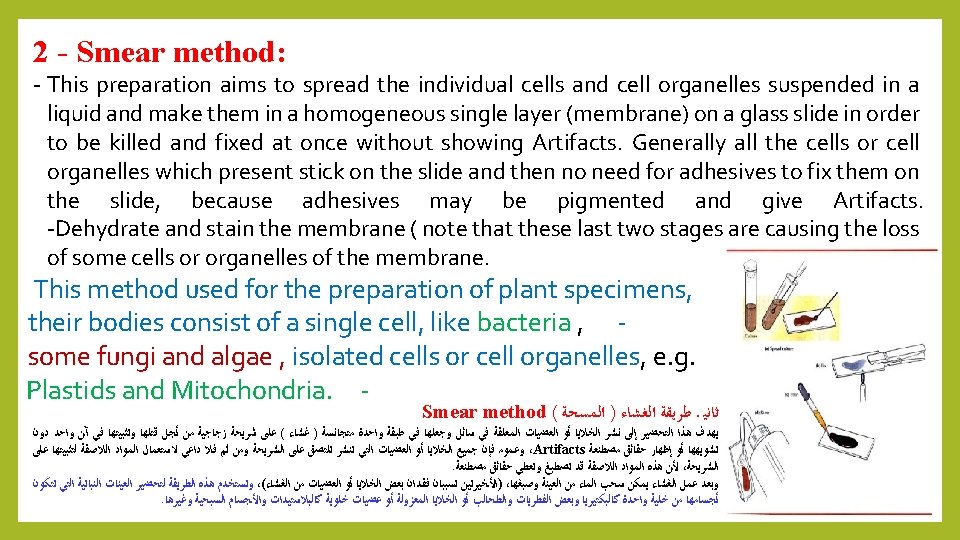




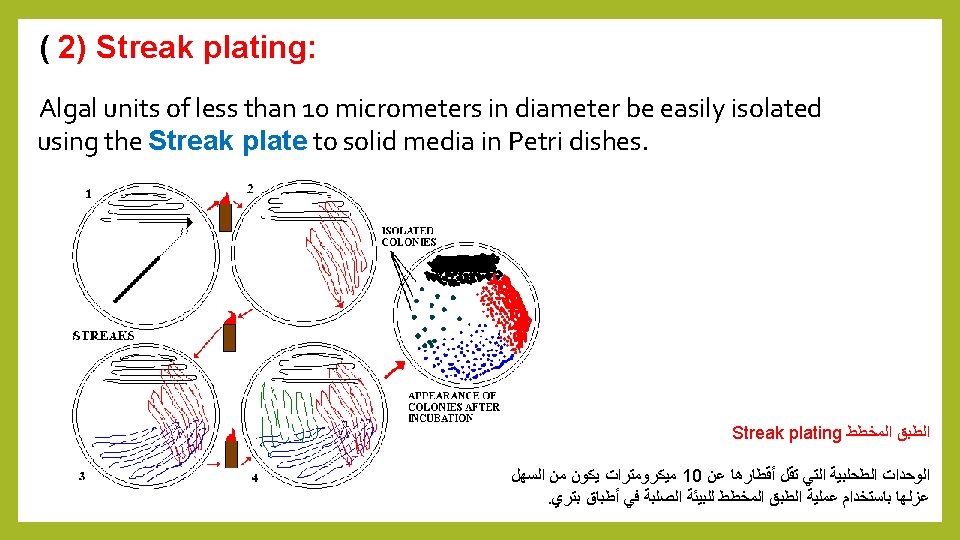



Purification: Often some physiological studies, Biochemistry on algae need pure culture. The isolation of algal units by Capillary pipette or Streak plate methods be used to liberated algal units from pollutants. Centrifugation and Ultrasonic vibrations can separate contaminated objects from algal units. 1 – Centrifugation: Purification process conducted by repeat washing. transfer algal units through the liquid environment and centrifugation 2 - Ultrasonic : This method is described by Brown and shows (1962). Used Ultrasonic water bath with a low-power 90 90 K cycles / second. Algal units will be separated from pollutants.



Stripped epidermis Method: 1 - Take a fresh leaf of a plant. 2. Stretch and break it by applying pressure. 3. While breaking it, keep it stretched gently so that some peel projects out from the cut. 4. Remove this peel and put it in a Petri dish filled with water and add a few drops of safranin. 5. Wait for few minutes and then transfer it onto a slide. 6. Gently place a cover slip over it. 7. Observe under microscope. When observed under Microscope. Outermost layer of cells called EPIDERMIS (dermal tissue) is seen. Leaf Epidermis
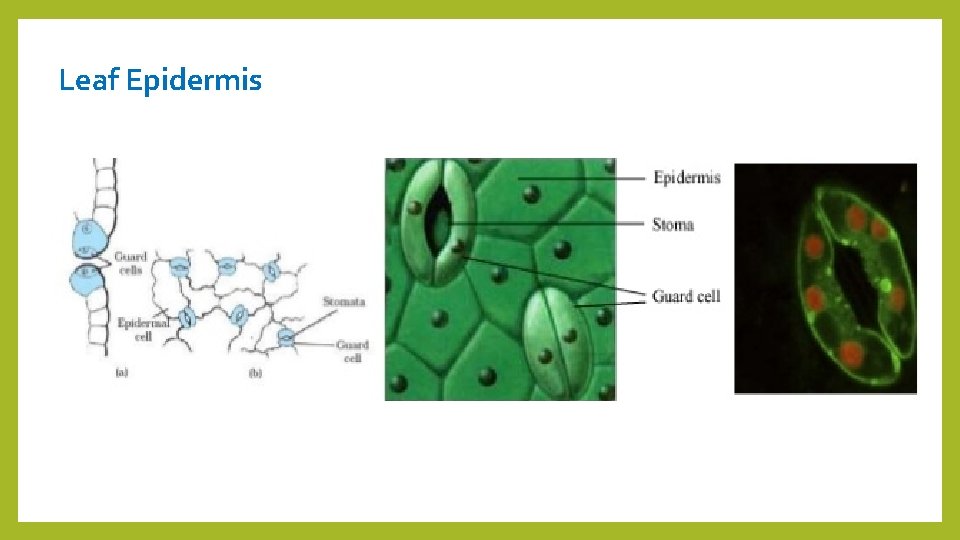
Leaf Epidermis
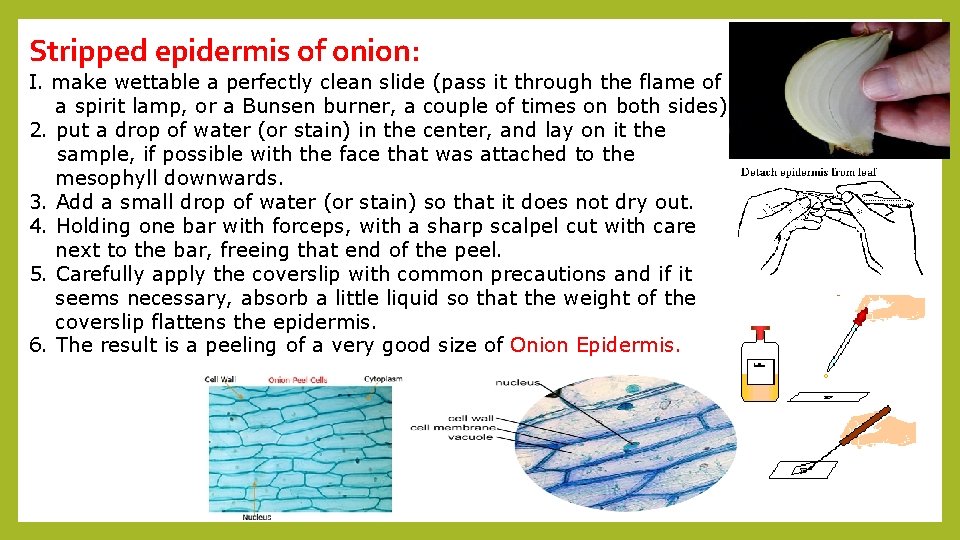
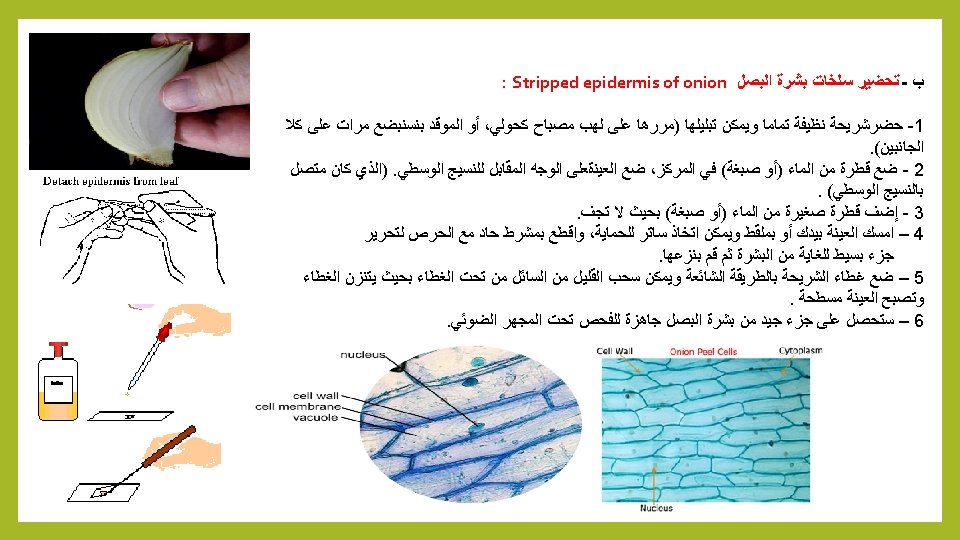
Stripped epidermis of onion: I. make wettable a perfectly clean slide (pass it through the flame of a spirit lamp, or a Bunsen burner, a couple of times on both sides). 2. put a drop of water (or stain) in the center, and lay on it the sample, if possible with the face that was attached to the mesophyll downwards. 3. Add a small drop of water (or stain) so that it does not dry out. 4. Holding one bar with forceps, with a sharp scalpel cut with care next to the bar, freeing that end of the peel. 5. Carefully apply the coverslip with common precautions and if it seems necessary, absorb a little liquid so that the weight of the coverslip flattens the epidermis. 6. The result is a peeling of a very good size of Onion Epidermis.

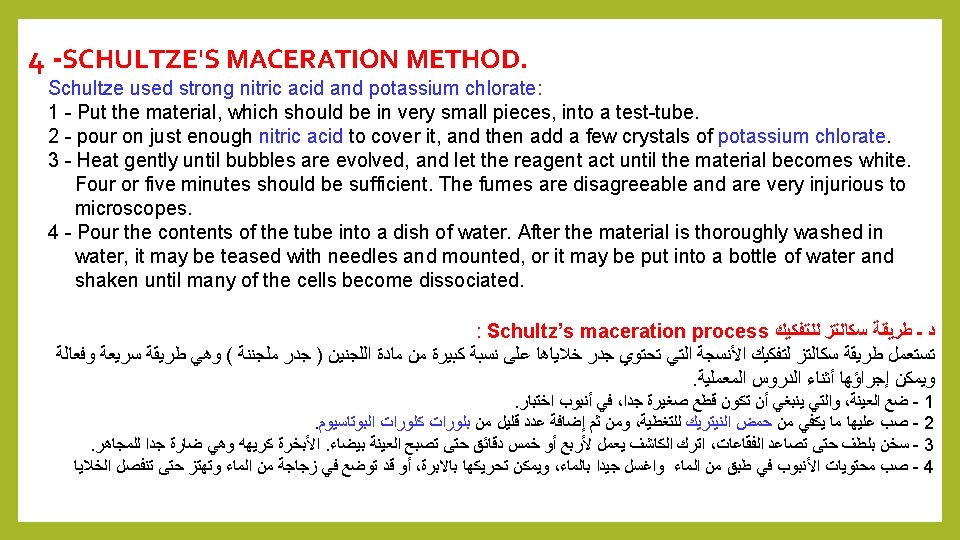


Quiz: What are the Sectioning Method or Microtomy steps? Answer: 1 – fixation. 2 – dehydration. 3 – embedding 4 – Sectioning 5 – Staining 6 - Mounting








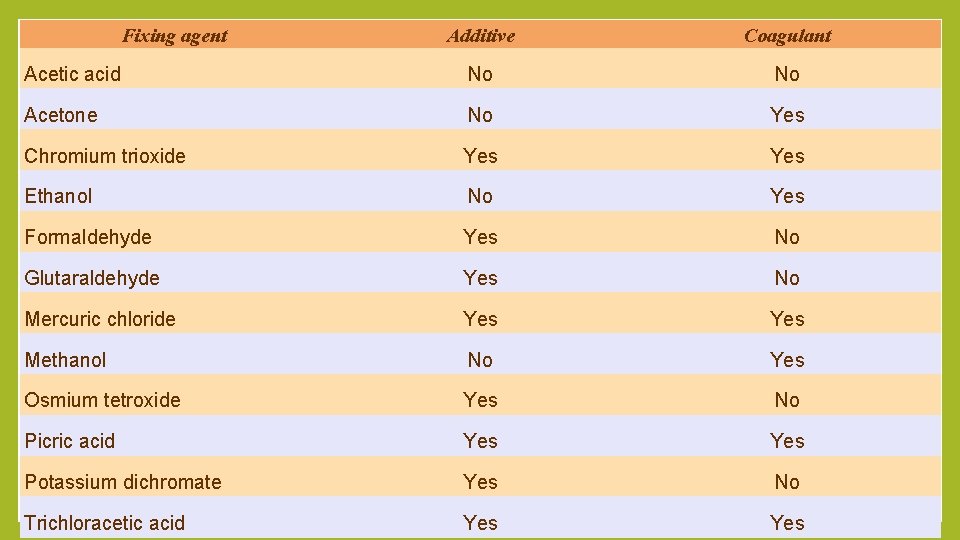
Fixing agent Additive Coagulant Acetic acid No No Acetone No Yes Chromium trioxide Yes Ethanol No Yes Formaldehyde Yes No Glutaraldehyde Yes No Mercuric chloride Yes Methanol No Yes Osmium tetroxide Yes No Picric acid Yes Potassium dichromate Yes No Trichloracetic acid Yes


Properties of an Ideal Fixative: 1. Prevents autolysis and bacterial decomposition. 2. Preserves sample tissues in their natural state and fix all components. 3. Make the cellular components insoluble to reagent used in tissue processing. 1. Preserves sample tissues volume. 2. Avoid excessive hardness of sample tissues 3. Allows enhanced staining of sample tissues 4. Should be non-toxic and non-allergic for user. 5. Should not be very expensive

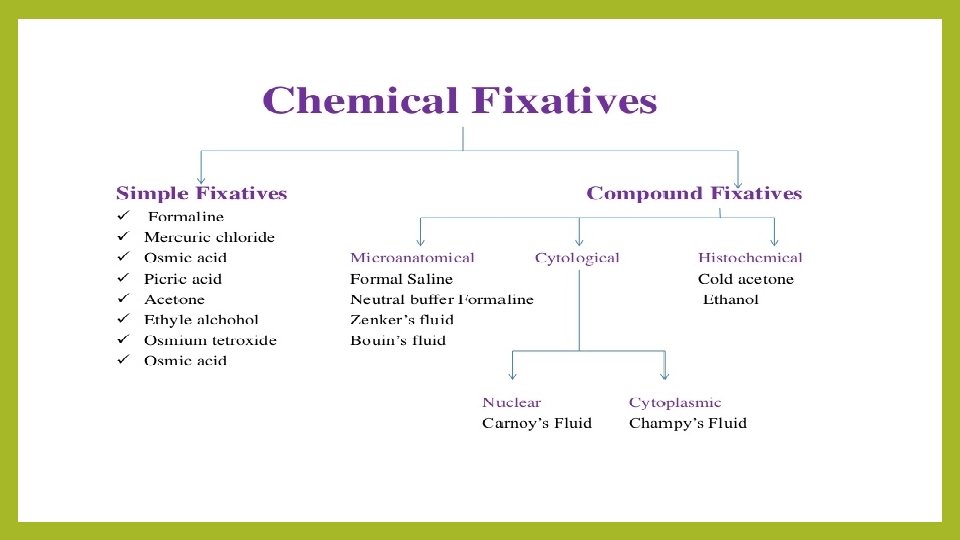







8 - Osmium Tetroxide: Osmium Tetroxide (OSO 4) yellow crystals, non-coagulant and when it dissolves in water become Osmic acid, a weak simple solutions is react with proteins and other components, forming intermolecular and intramolecular cross-links resulting in a better retention of the cellular organization. . It characterized by slow penetration to the sample parts, it also makes the sample soft is difficult to cut. It does not cause shrinking to the sample, it must be removed from the sample after the fixation process by washed with running water. It used as 1% aqueous solution. : Osmium Tetroxide ﺍﻷﻮﺯﻣﻴﻮﻡ ﺃﻜﺴﻴﺪ ﺭﺍﺑﻊ ـ 8 ﺍﻟﻤﺤﺎﻟﻴﻞ ﻭﻣﻦ ﺿﻌﻴﻒ ﺣﻤﺾ ﻭﻫﻮ ، ﺍﻷﻮﺯﻣﻴﻚ ﺣﻤﺾ ﺗﻜﻮﻥ ﺑﺎﻟﻤﺎﺀ ﺫﻭﺑﺎﻧﻬﺎ ﻭﻋﻨﺪ ، ﺻﻔﺮﺍﺀ ﺑﻠﻮﺭﺍﺕ ﻣﻦ ( OSO 4 ) ﺍﻷﺰﻣﻴﻮﻡ ﺃﻜﺴﻴﺪ ﺭﺍﺑﻊ ﻳﺘﻜﻮﻥ ﺑﻌﺪ ﺍﻟﻌﻴﻨﺔ ﻣﻦ ﺇﺯﺍﻟﺘﻪ ﻭﻳﺠﺐ ، ﺍﻟﻌﻴﻨﺔ ﺍﻧﻜﻤﺎﺵ ﻳﺴﺒﺐ ﻭﻻ ، ﻗﻄﻌﻬﺎ ﻳﺼﻌﺐ ﻃﺮﻳﺔ ﻳﺠﻌﻠﻬﺎ ﺃﻨﻪ ﻛﻤﺎ ، ﺍﻟﻌﻴﻨﺔ ﺃﺠﺰﺍﺀ ﺇﻟﻰ ﻧﻔﺎﺫﻩ ﺑﺒﻄﺀ ﻭﻳﺘﻤﻴﺰ ، ﺍﻟﻤﺨﺜﺮﺓ ﻏﻴﺮ ﺍﻷﻮﻟﻴﺔ . ( ٪ 1 ﺑﺘﺮﻛﻴﺰ ﻣﺎﺋﻲ ﻛﻤﺤﻠﻮﻝ ﻳﺴﺘﻌﻤﻞ ﻭﻫﻮ ) ، ﺍﻟﺠﺎﺭﻱ ﺑﺎﻟﻤﺎﺀ ﺑﻐﺴﻠﻪ ﺍﻟﺘﺜﺒﻴﺖ



1 - Formalin– Acitic acid–Alcohol: FAA Formalin–acetic acid–alcohol (FAA) is a good general fixative to plant samples. Compared to other fixatives. Sample penetration is not particularly fast. Due to the presence of alcohols, shrinkage may occur. You may vary the amount of acetic from 2 to 6% to modulate shrinkage and better preserve chromatin structure. Increase concentration of acetic to induce greater tissue swelling and to counteract alcohol shrinkage. Generally, samples are killed and hardened within 12– 24 h. The fixative is stable and does not induce hardening, so samples may be stored in this solution. The wooden parts of stems and roots the fixation times will be one weeks. The concentration of 50% ethyl alcohol used with soft samples, while 70% concentration of ethyl alcohol used with woody samples. After the fixation process, the samples should be washed twice to three times with 50% or 70% ethyl alcohol according to fixative concentration Ethyl alcohol 95% 90 ml Formalin 5 ml Glacial acetic acid 5 ml

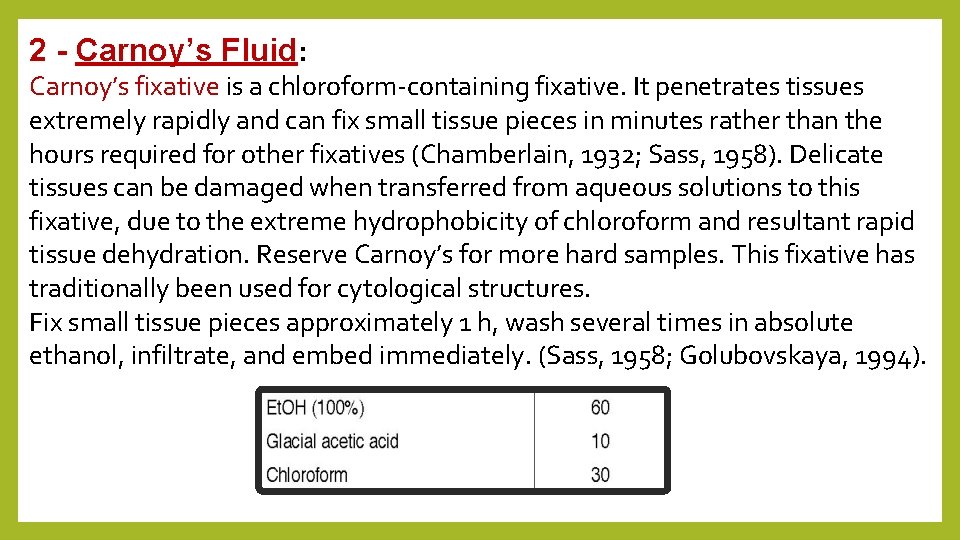
2 - Carnoy’s Fluid: Carnoy’s fixative is a chloroform-containing fixative. It penetrates tissues extremely rapidly and can fix small tissue pieces in minutes rather than the hours required for other fixatives (Chamberlain, 1932; Sass, 1958). Delicate tissues can be damaged when transferred from aqueous solutions to this fixative, due to the extreme hydrophobicity of chloroform and resultant rapid tissue dehydration. Reserve Carnoy’s for more hard samples. This fixative has traditionally been used for cytological structures. Fix small tissue pieces approximately 1 h, wash several times in absolute ethanol, infiltrate, and embed immediately. (Sass, 1958; Golubovskaya, 1994).

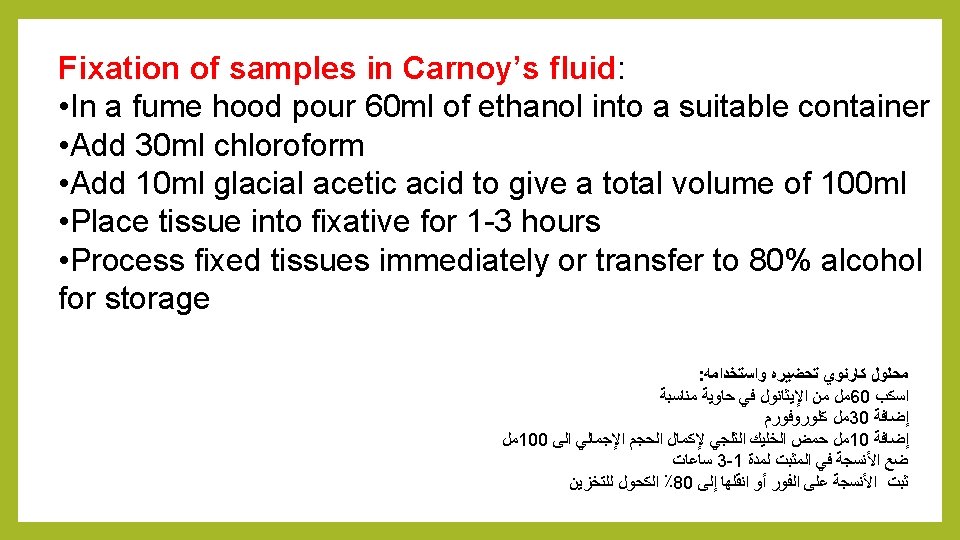



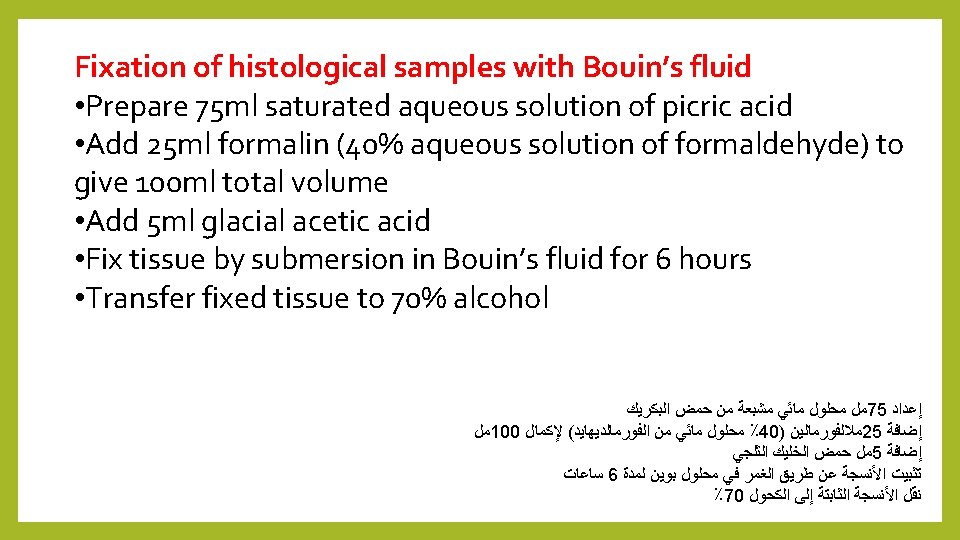


6 - Zenker’s fluid fixation: Contributed by Martin Fitzpatrick, University of Birmingham, United Kingdom Fixation with Zenker’s fluid • Add 950 ml distilled water to a suitable container • Add 25 g potassium dichromate • Add 50 g mercuric chloride • Add 50 g glacial acetic acid • Mix thoroughly to dissolve • Fix samples for 4 -24 hours • Following fixation wash samples overnight in running tap water prior to processing


7 - Zirkle 's Fluid: This is a good routine fixative giving excellent morphological preservation, although it is not used as much as it was in the past due to the mercuric chloride content. It consists of: § 400 ml of distilled Water § 2. 5 g Potassium bichromate § 2. 5 g Ammonium bichromate § 2 g of Cupper sulphate The sample should be placed in this solution for 24 to 48 hours and then washed subsequently with water several times and then washed with water.




10 - Acetic alcohol fixative Acetic alcohol (Farmer) : It uses for cytological studies especially chromosomes, consisting of absolute alcohol and glacial acetic acid of 3: 1, respectively, for example: 75 ml of absolute Ethyl alcohol. 25 ml Glacial acetic acid. And placed the sample for 24 hours chromosomes appears clear, but non-existent or fade cytoplasmic organelles. Acetic alcohol fixation: Contributed by Martin Fitzpatrick, University of Birmingham, United Kingdom. Fixative for smears. . • Add 3 ml glacial acetic acid to 100 ml 95% methanol. • Fix section in solution for 1 minute. • Rinse section in distilled water prior to staining.

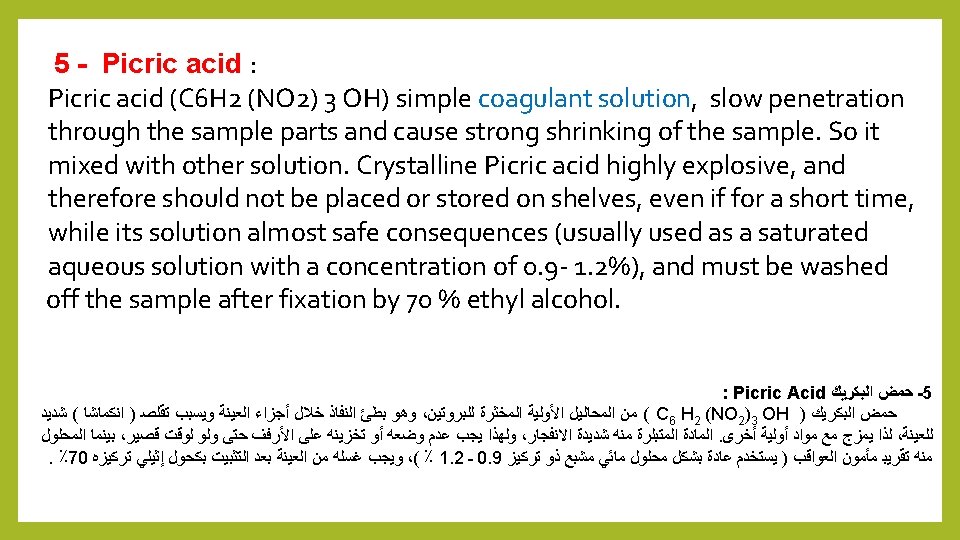
11 - Allen Fluid's fixative: This solution uses as a fixative to most plant tissues that do not contain a large proportion of the wooden elements, particularly the floral buds and developing apexes. It consists of the following simple materials: 75 ml saturated aqueous solution of picric acid Pecric acid 1 -25 ml Formalin 2 - 5 ml Glacial acetic acid 3 - 1. 5 ml Chromic acid 4 - 2 g of urea (urine) The sample must remain in this solution between 4 to 16 hours, and then transferred to 70% ethyl alcohol, several times until the yellow color of picric acid disappeared, preferably prepared when to be use due to the speed damaged.


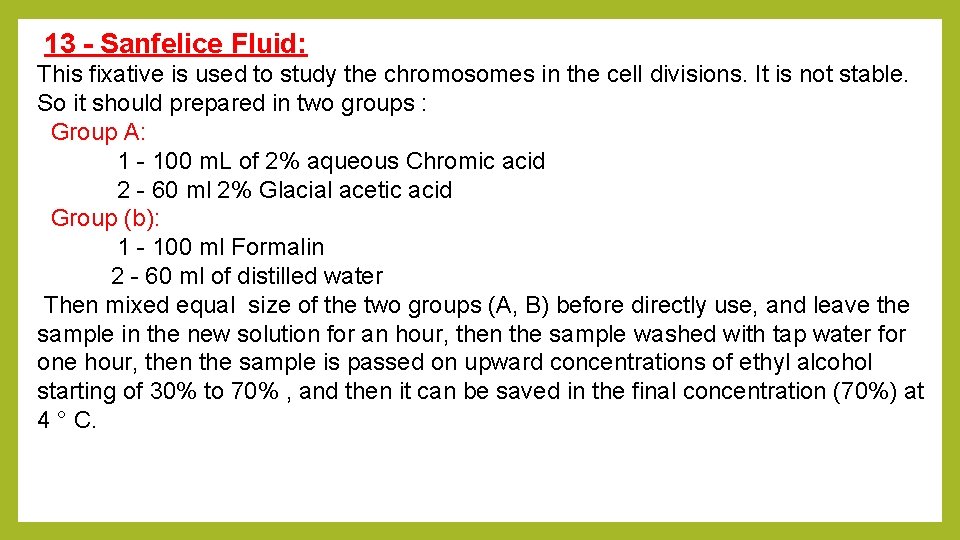
13 - Sanfelice Fluid: This fixative is used to study the chromosomes in the cell divisions. It is not stable. So it should prepared in two groups : Group A: 1 - 100 m. L of 2% aqueous Chromic acid 2 - 60 ml 2% Glacial acetic acid Group (b): 1 - 100 ml Formalin 2 - 60 ml of distilled water Then mixed equal size of the two groups (A, B) before directly use, and leave the sample in the new solution for an hour, then the sample washed with tap water for one hour, then the sample is passed on upward concentrations of ethyl alcohol starting of 30% to 70% , and then it can be saved in the final concentration (70%) at 4 ° C.




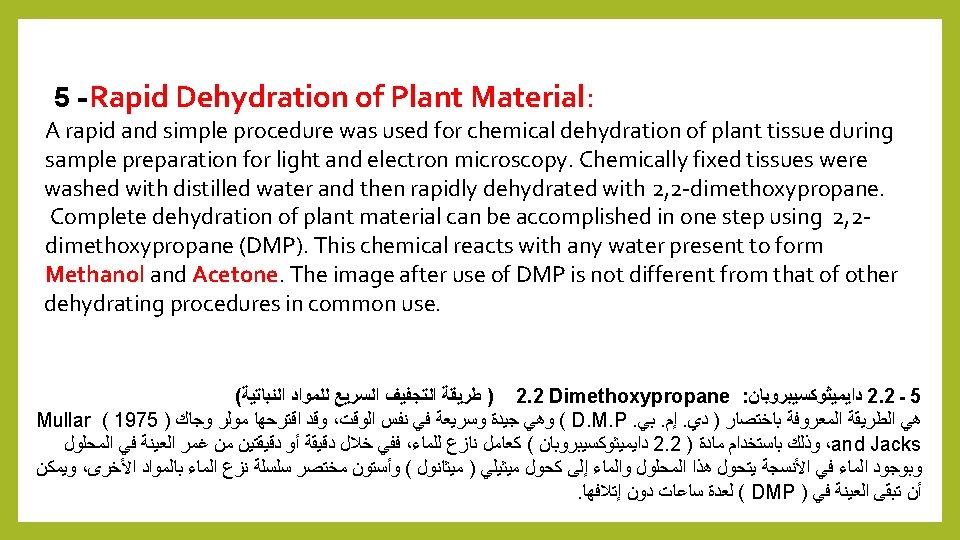
Dehydration Because melted is hydrophobic (immiscible with water), most of the water in a specimen must be removed before it can be infiltrated with wax. This process is commonly carried out by immersing specimens in a series of ethanol (alcohol) solutions of increasing concentration until pure, water-free alcohol is reached. Ethanol is miscible with water in all proportions so that the water in the specimen is progressively replaced by the alcohol. A series of increasing concentrations is used to avoid excessive distortion of the tissue. ﺍﻟﻨﻘﻴﺔ ﺣﺘﻰ ﺍﻟﺘﺮﻛﻴﺰ ﻓﻲ ﻣﺘﺰﺍﻳﺪﻩ ﻣﺤﺎﻟﻴﻞ ( )ﺍﻟﻜﺤﻮﻝ ﺍﻹﻳﺜﺎﻧﻮﻝ ﻣﻦ ﺳﻠﺴﻠﺔ ﻓﻲ ﺍﻟﻌﻴﻨﺎﺕ ﻏﻤﺮ ﻃﺮﻳﻖ ﻋﻦ ﻋﺎﺩﺓ ﺍﻟﻌﻤﻠﻴﺔ ﻫﺬﻩ ﺗﻨﻔﻴﺬ ﻳﺘﻢ . ﺑﺎﻟﺸﻤﻊ ﺗﺸﺮﻳﺒﻬﺎ ﻗﺒﻞ ﻋﻴﻨﺔ ﻣﻦ ﺍﻟﻤﺎﺀ ﻣﻌﻈﻢ ﺇﺯﺍﻟﺔ ﻳﺠﺐ . ﻟﻸﻨﺴﺠﺔ ﺍﻟﻤﻔﺮﻁ ﺗﺸﻮﻳﻪ ﻟﺘﺠﻨﺐ ﺍﻟﻤﺘﺰﺍﻳﺪﺓ ﺍﻟﺘﺮﻛﻴﺰﺍﺕ ﻣﻦ ﺳﻠﺴﻠﺔ ﺍﺳﺘﺨﺪﺍﻡ ﻳﺘﻢ . ﺍﻟﻤﺎﺀ ﻣﻦ ﺧﺎﻟﻲ A- Fluids not dissolve waxes: 1 - Ethyl alcohol 4 - Glycerin 2 - Isopropyle alcohol 5 - 2. 2 Dimethoxypropane 3 - Acetone

1 -Fluids not dissolve waxes: 1 - Ethyl alcohol : ﻟﻠﺸﻤﻊ ﻣﺬﻳﺒﺔ ﻏﻴﺮ ﻣﺤﺎﻟﻴﻞ ـ ﺃﻮﻻ Commercial solution is 95% ethyl alcohol, while the absolute is completely free of water, usually used 95% ethyl alcohol for dehydration of the plant samples 2 - Isopropyle alcohol It used as Ethyl alcohol 3 - Acetone It does not cause any problems, it is always the alternative of ethyl alcohol 4 - Glycerin Used with algae and soft thin samples, and the high boiling point of glycerin limit water loss by evaporation.


2 - Fluids dissolve wax : ﻟﻠﺸﻤﻊ ﻣﺬﻳﺒﺔ ﻣﺤﺎﻟﻴﻞ ـ ﺛﺎﻧﻴ 1 - Normal Butyl Alcohol ( Butanol ) 2 - Tertiary Butyl Alcohol 2 - Dioxane 1 - Normal Butyl Alcohol ( Butanol ) : Wash the sample placed in the fixative with water, then dehydrate by ethyl alcohol until the concentration of 30%, then the sample is moved to No. 1 in the Table below, and then follow the other concentrations to the end of the number (8) in the same table. Water 70 60 45 30 20 10 0 0 ethyl alcohol 20 25 30 30 25 20 15 0 Normal Butyl Alcohol 10 15 25 40 55 70 85 100 No 1 2 3 4 5 6 7 8 : ﻟﻠﺸﻤﻊ ﻣﺬﻳﺒﺔ ﻣﺤﺎﻟﻴﻞ ـ ﺛﺎﻧﻴ : ( ﺑﻴﻮﺗﺎﻧﻮﻝ ) ﺍﻟﻌﺎﺩﻱ ﺍﻟﻜﺤﻮﻝ ﺑﻮﺗﺎﻳﻞ ـ 1 Normal Butyl Alcohol ( Butanol ) : ﺍﻹﺛﻴﻠﻲ ﺑﺎﻟﻜﺤﻮﻝ ﺍﻟﻤﺎﺀ ﻳﺴﺤﺐ ﺛﻢ ، ﺑﺎﻟﻤﺎﺀ ﺍﻟﻤﺜﺒﺖ ﻓﻲ ﺍﻟﻤﻮﺿﻮﻋﺔ ﺍﻟﻌﻴﻨﺔ ﺗﻐﺴﻞ ﺛﻢ ﻭﻣﻦ ، ﺍﻟﺘﺎﻟﻲ (5) ﺭﻗﻢ ﺍﻟﺠﺪﻭﻝ ﻓﻲ (1) ﺭﻗﻢ ﺇﻟﻰ ﺍﻟﻌﻴﻨﺔ ﺗﻨﻘﻞ ﺛﻢ ، ٪ 30 ﺗﺮﻛﻴﺰ ﺣﺘﻰ . ﻧﻔﺴﻪ ﺍﻟﺠﺪﻭﻝ ﻓﻲ (8) ﺍﻟﺮﻗﻢ ﻧﻬﺎﻳﺔ ﺇﻟﻰ ﺍﻷﺨﺮﻯ ﺍﻟﺘﺮﺍﻛﻴﺰ ﺗﺘﺒﻊ
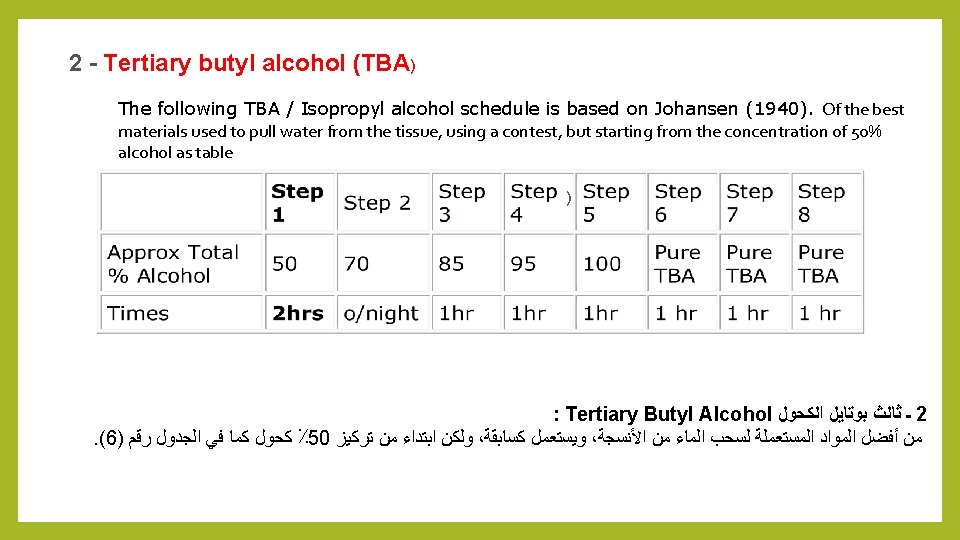
2 - Tertiary butyl alcohol (TBA) The following TBA / Isopropyl alcohol schedule is based on Johansen (1940). Of the best materials used to pull water from the tissue, using a contest, but starting from the concentration of 50% alcohol as table ) : Tertiary Butyl Alcohol ﺍﻟﻜﺤﻮﻝ ﺑﻮﺗﺎﻳﻞ ﺛﺎﻟﺚ ـ 2 . (6) ﺭﻗﻢ ﺍﻟﺠﺪﻭﻝ ﻓﻲ ﻛﻤﺎ ﻛﺤﻮﻝ ٪ 50 ﺗﺮﻛﻴﺰ ﻣﻦ ﺍﺑﺘﺪﺍﺀ ﻭﻟﻜﻦ ، ﻛﺴﺎﺑﻘﺔ ﻭﻳﺴﺘﻌﻤﻞ ، ﺍﻷﻨﺴﺠﺔ ﻣﻦ ﺍﻟﻤﺎﺀ ﻟﺴﺤﺐ ﺍﻟﻤﺴﺘﻌﻤﻠﺔ ﺍﻟﻤﻮﺍﺩ ﺃﻔﻀﻞ ﻣﻦ


What is The process of Dehydration? And its methods of using paraffin wax? It is done gradually in a series of different solutions. There are two Methods A- Dehydrated solutions does not melt the paraffin wax 1 - Ethyl alcohol 4 - Glycerin 2 - Isopropyle alcohol 5 - 2. 2 Dimethoxypropane 3 - Acetone B - Dehydrated solutions melt the paraffin wax. 1 - Normal Butyl Alcohol ( Butanol ) 2 - Tertiary Butyl Alcohol 2 - Dioxane
- Slides: 136