Plant Breeding an Overview Objective 1 know basic

Plant Breeding – an Overview

Objective 1: know basic plant genetics and breeding terminology
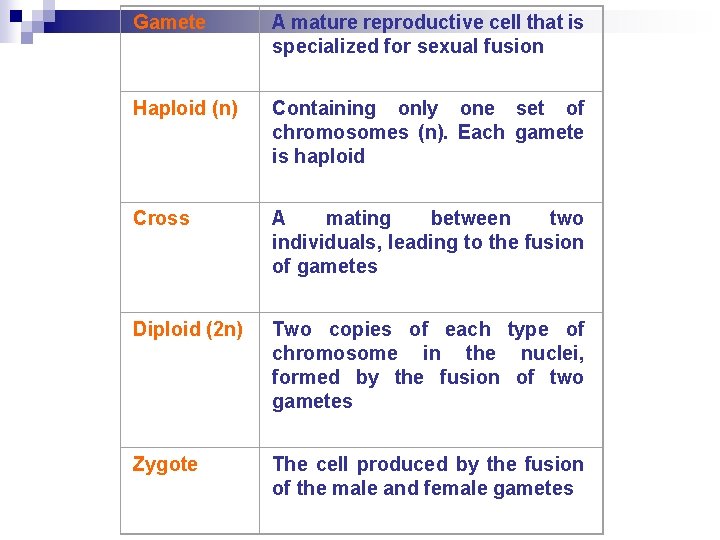
Gamete A mature reproductive cell that is specialized for sexual fusion Haploid (n) Containing only one set of chromosomes (n). Each gamete is haploid Cross A mating between two individuals, leading to the fusion of gametes Diploid (2 n) Two copies of each type of chromosome in the nuclei, formed by the fusion of two gametes Zygote The cell produced by the fusion of the male and female gametes
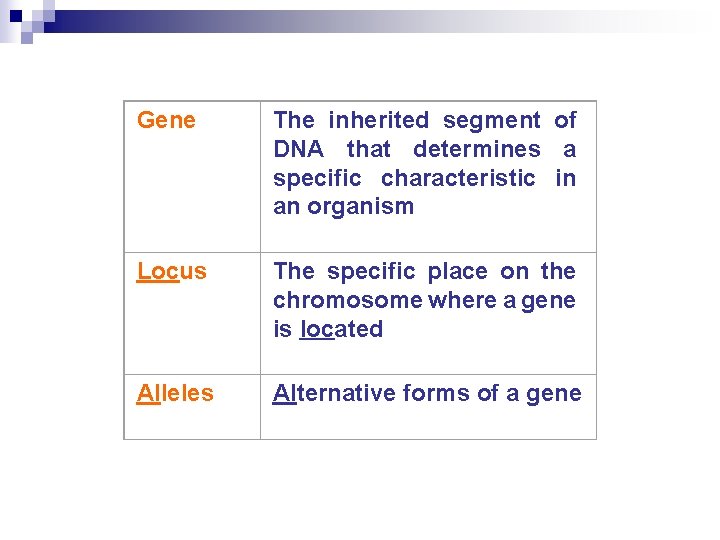
Gene The inherited segment of DNA that determines a specific characteristic in an organism Locus The specific place on the chromosome where a gene is located Alleles Alternative forms of a gene
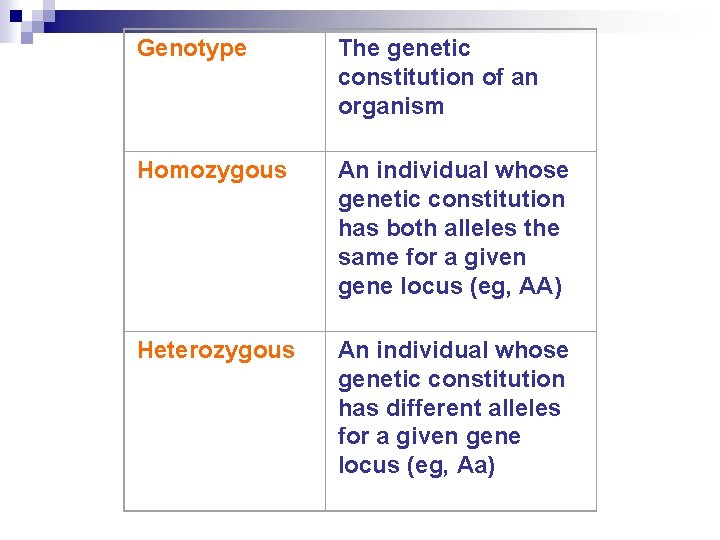
Genotype The genetic constitution of an organism Homozygous An individual whose genetic constitution has both alleles the same for a given gene locus (eg, AA) Heterozygous An individual whose genetic constitution has different alleles for a given gene locus (eg, Aa)

Homogeneous A population of individuals having the same genetic constitution (eg, a field of pure-line soybean; a field of hybrid corn) Heterogeneous A population of individuals having different genetic constitutions Phenotype The physical manifestation of a genetic trait that results from a specific genotype and its interaction with the environment

What is Plant Breeding? n The genetic adjustment of plants to the service of humankind ---Sir Otto Frankel Source: http: //www. ars. usda. gov/is/graphics/photos/

Objective 2: know why plant breeding is important and useful Several examples in soybean
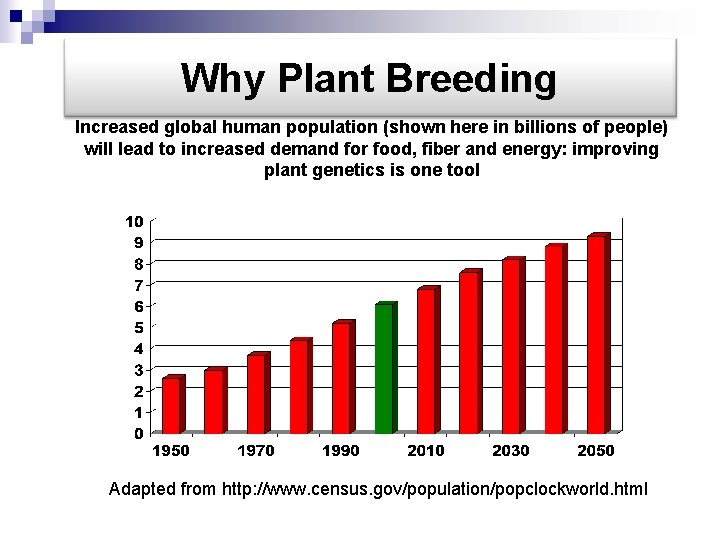
Why Plant Breeding Increased global human population (shown here in billions of people) will lead to increased demand for food, fiber and energy: improving plant genetics is one tool Adapted from http: //www. census. gov/population/popclockworld. html

Plant Breeding Targets 1. Yield Source: USB photo disc 0976

Plant breeding has contributed to more than 50% of increased USA crop productivity during the last 30 years Source: http: //www. ars. usda. gov/is/graphics/photos/

Plant Breeding Targets Improved product quality Source: http: //www. ars. usda. gov/is/graphics/photos/

• Hydrogenation: flavor and oxidative stability • Trans fats: health issues • FDA label mandate cis form saturated H H C C Hydrogenation C C H H trans form H ; C C H (Source: Wilson, 2004)

Plant Breeding Targets 3. Pest and Disease Resistance Soybean sudden death syndrome

Joint Germplasm Release (Drs. Arelli, Pantalone, Allen, Mengistu) USDA-ARS and Tennessee Agricultural Exp. Stn. Release of JTN-5303 Soybean Resistant to multiple diseases: Soybean cyst nematode Sudden death syndrome Stem canker Frogeye leaf spot Charcoal rot
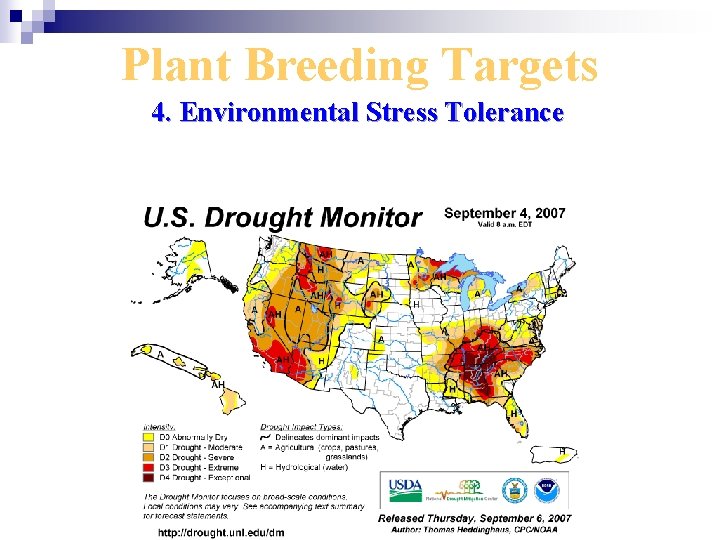
Plant Breeding Targets 4. Environmental Stress Tolerance

Plant Breeding Targets 5. Ease of Management Deployment of transgenic traits (e. g. , transfer of herbicide resistant genes in commercial varieties)

Plant Breeding Targets 6. Adaptation to Mechanization Source: http: //www. ars. usda. gov/is/graphics/photos/

Plant Breeding Targets 7. Environmental sustainability Conservation Tillage Source: http: //www. ars. usda. gov/is/graphics/photos/

Objective 3: know the basic principles of plant breeding Importance of genetic variation and selection

What are the causes of biological variation observed in plants? 1. Genetic causes (mode of inheritance) n single genes n multiple genes 2. Environmental 3. Gx. E: the interaction between the genotype of the plant and the environment in which it grows

A plant breeder needs to: be observant of phenotypic differences among plants n understand the genetics n have the imagination to visualize final product n foresight to predict demand for future plant products n

Plant selections to improve plant traits are made by assessing plant phenotypes n In plants, examples include: Ø plant height Ø plant and leaf morphology Ø biomass yield Ø seed yield Ø chemical composition of plant tissues and seeds
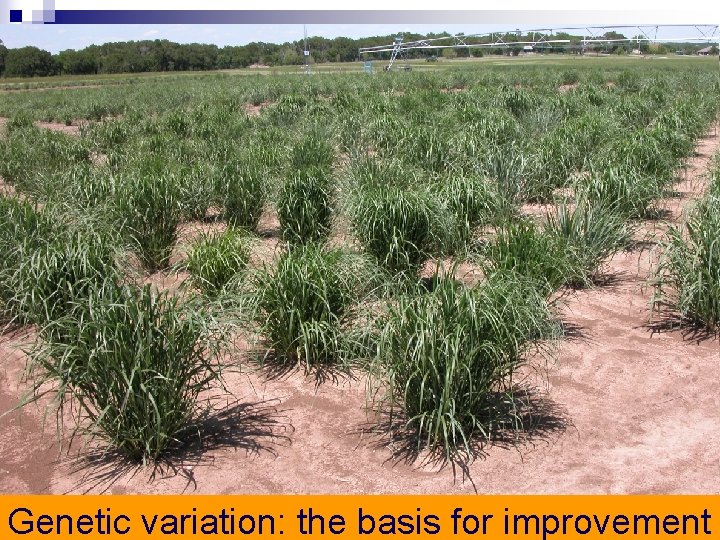
Genetic variation: the basis for improvement
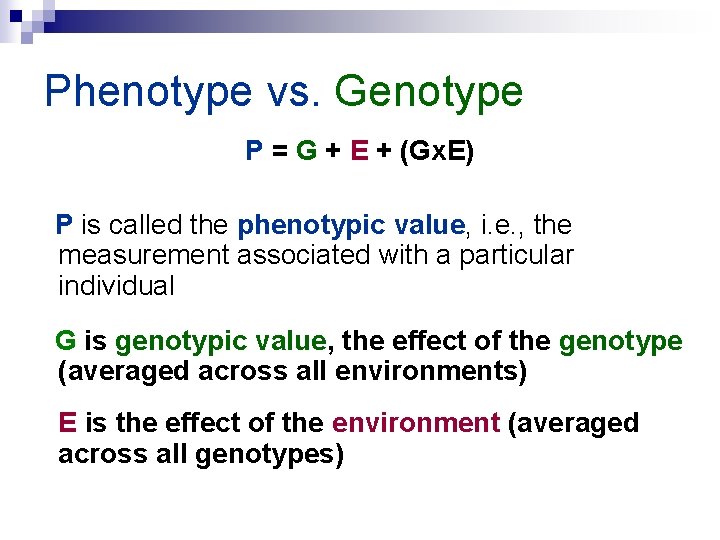
Phenotype vs. Genotype P = G + E + (Gx. E) P is called the phenotypic value, i. e. , the measurement associated with a particular individual G is genotypic value, the effect of the genotype (averaged across all environments) E is the effect of the environment (averaged across all genotypes)
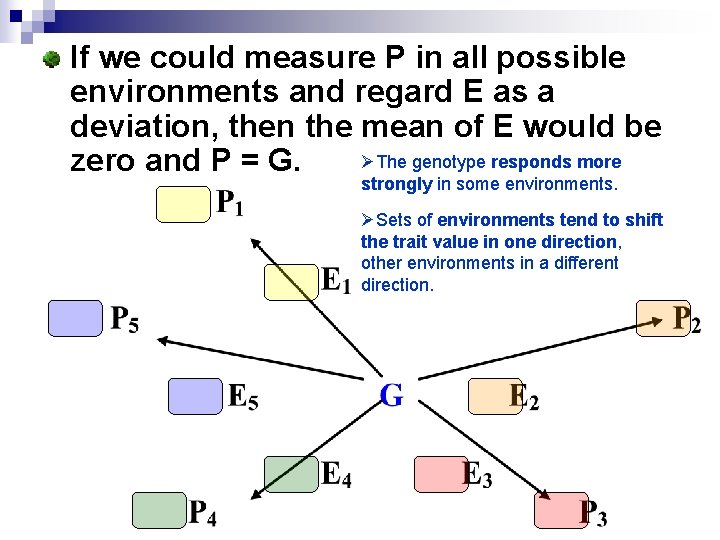
If we could measure P in all possible environments and regard E as a deviation, then the mean of E would be ØThe genotype responds more zero and P = G. strongly in some environments. ØSets of environments tend to shift the trait value in one direction, other environments in a different direction.
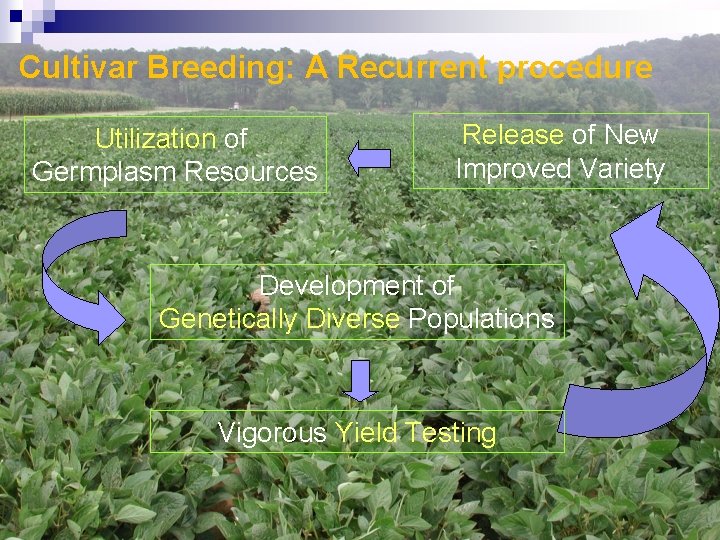
Cultivar Breeding: A Recurrent procedure Utilization of Germplasm Resources Release of New Improved Variety Development of Genetically Diverse Populations Vigorous Yield Testing

Controlled Cross Pollination Parent 1 × Parent 2 Stigma

Objective 4: know some basic plant breeding methods and strategies

How do we breed improved crop cultivars? 1. Inheritance of trait
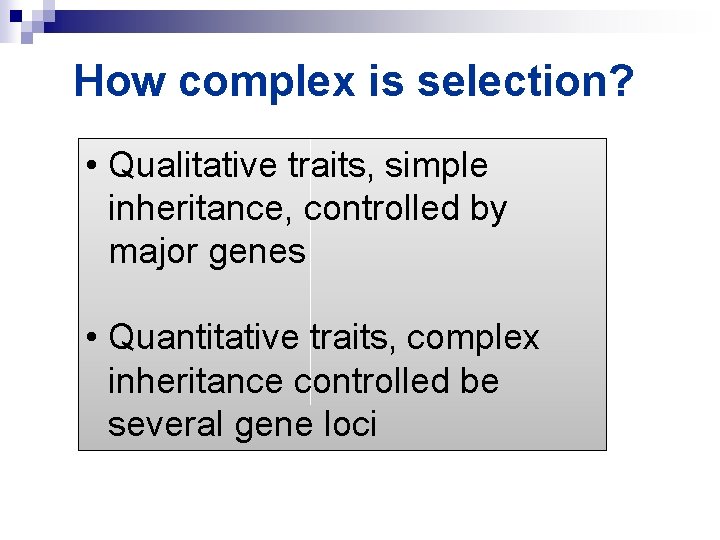
How complex is selection? • Qualitative traits, simple inheritance, controlled by major genes • Quantitative traits, complex inheritance controlled be several gene loci

Qualitative traits v Classified into discrete classes v Individuals in each class counted v Some environmental influence on phenotype v Controlled by a few (<3) major genes
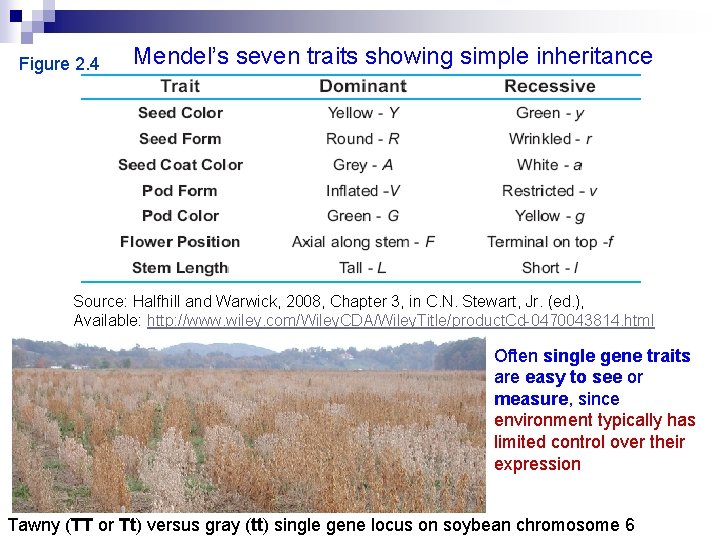
Figure 2. 4 Mendel’s seven traits showing simple inheritance Source: Halfhill and Warwick, 2008, Chapter 3, in C. N. Stewart, Jr. (ed. ), Available: http: //www. wiley. com/Wiley. CDA/Wiley. Title/product. Cd-0470043814. html Often single gene traits are easy to see or measure, since environment typically has limited control over their expression Tawny (TT or Tt) versus gray (tt) single gene locus on soybean chromosome 6
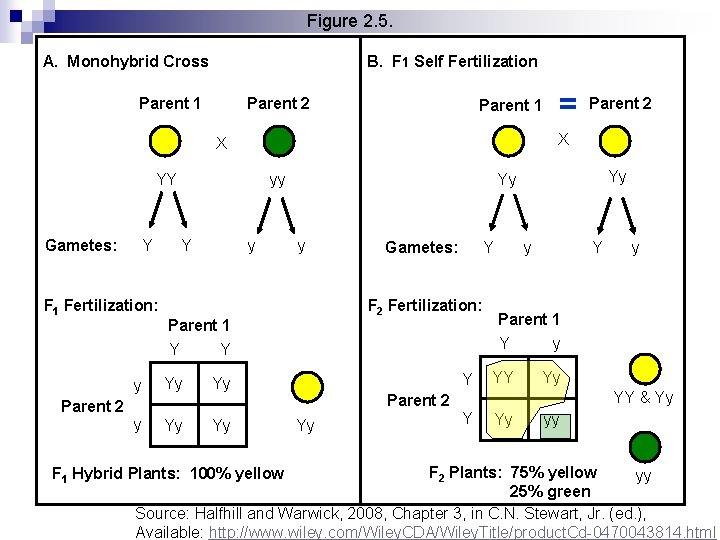
Figure 2. 5. A. Monohybrid Cross B. F 1 Self Fertilization Parent 1 Parent 2 Parent 1 YY Y F 1 Fertilization: yy Y y Yy Yy y Gametes: Y F 2 Fertilization: Parent 1 Y Y y Parent 2 X X Gametes: = Y y Y Parent 1 Y y YY Yy YY & Yy Parent 2 Yy y Y Yy yy F 2 Plants: 75% yellow yy 25% green Source: Halfhill and Warwick, 2008, Chapter 3, in C. N. Stewart, Jr. (ed. ), Available: http: //www. wiley. com/Wiley. CDA/Wiley. Title/product. Cd-0470043814. html F 1 Hybrid Plants: 100% yellow

Gene and Genotype Frequencies Example: Self pollinated diploid species Upon selfing F 2 population; 25% homozygous ‘YY’ will produce only ‘YY’ genotypes, and 25% homozygous ‘cc’ will produce only ‘yy’ genotypes. So only ‘Yy’ will segregate to produce genotypes in proportion of 0. 25 (YY): 0. 50: (Yy): 0. 25(yy). F 2 population: 0. 25(YY ) 0. 50 (Cc) 0. 25 (cc ) YY 0. 25 Produce all CC plants Resulting F 3 population will have 0. 25 + ½ (0. 25) = 0. 375 CC plants Yy Yy 0. 50 Segregate into 0. 25(CC ) 0. 50% (Cc) and 0. 25 (cc) ½ (0. 50) = 0. 25 Cc plants yy 0. 25 Produce all cc plants ½ (0. 25) + (0. 25) = 0. 375 cc plants
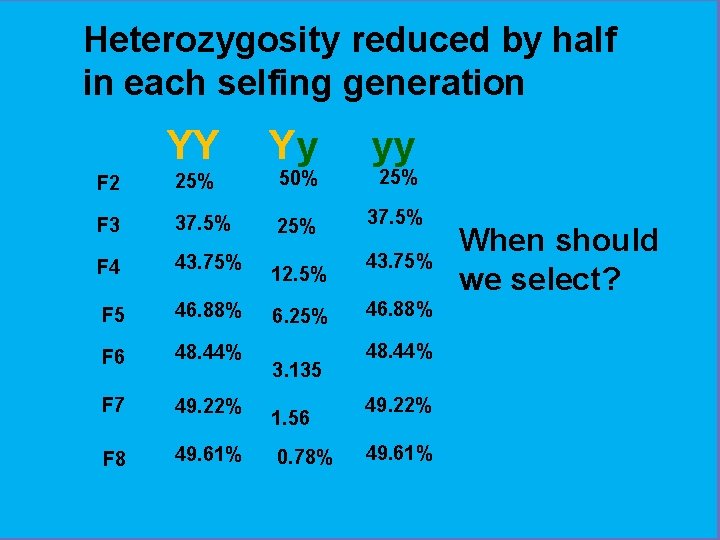
Heterozygosity reduced by half in each selfing generation YY Yy yy 37. 5% F 2 25% 50% F 3 37. 5% 25% F 4 43. 75% F 5 46. 88% F 6 48. 44% F 7 49. 22% F 8 49. 61% 12. 5% 6. 25% 3. 135 1. 56 0. 78% 25% 43. 75% 46. 88% 48. 44% 49. 22% 49. 61% When should we select?

Questions based on F 5 single plant derived progeny rows from one population formed from crossing two pure line parents:

Selfing a double het (Aa. Bb × Aa. Bb) produces a 9: 3: 3: 1 phenotypic ratio only if trait governed by complete dominance Freq Genotype Phenotypic Ratio Underlying Genotypes 9 AABB = AABb = Aa. BB = Aa. Bb 1/16 AABB 2/16 AABb 1/16 AAbb 2/16 Aa. BB 3 AAbb = Aabb 4/16 Aa. Bb 3 aa. BB = aa. Bb 2/16 Aabb 1 aabb 1/16 aa. BB 2/16 aa. Bb 1/16 aabb Note: only 1 out of 16 is homozygous favorable allele for both gene loci
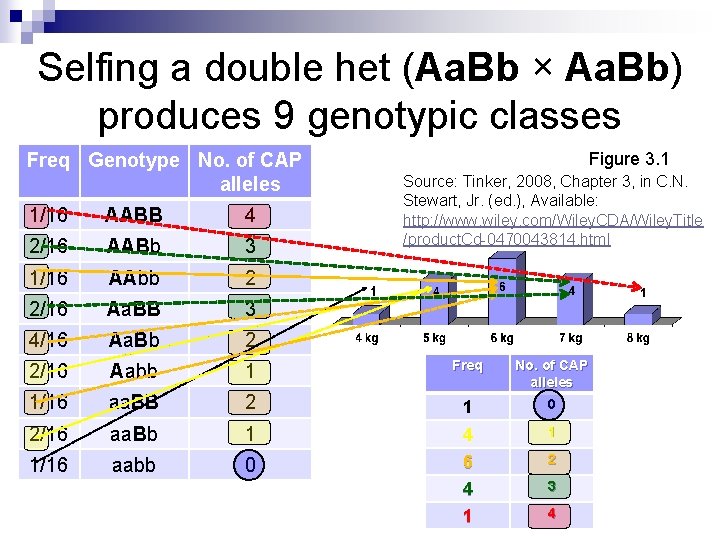
Selfing a double het (Aa. Bb × Aa. Bb) produces 9 genotypic classes Freq Genotype No. of CAP alleles Figure 3. 1 Source: Tinker, 2008, Chapter 3, in C. N. Stewart, Jr. (ed. ), Available: http: //www. wiley. com/Wiley. CDA/Wiley. Title /product. Cd-0470043814. html 1/16 AABB 4 2/16 AABb 3 1/16 AAbb 2 2/16 Aa. BB 3 4/16 Aa. Bb 2 2/16 Aabb 1 Freq No. of CAP alleles 1/16 aa. BB 2 1 0 2/16 aa. Bb 1 1 1/16 aabb 0 4 6 4 1 3 2 4
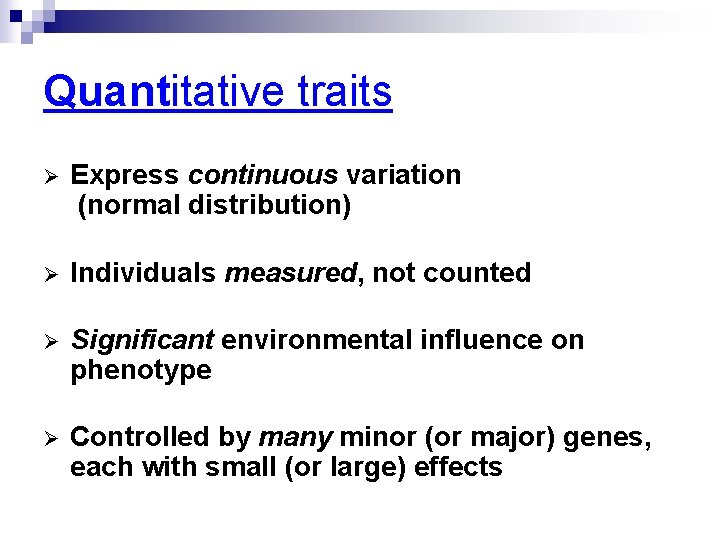
Quantitative traits Ø Express continuous variation (normal distribution) Ø Individuals measured, not counted Ø Significant environmental influence on phenotype Ø Controlled by many minor (or major) genes, each with small (or large) effects
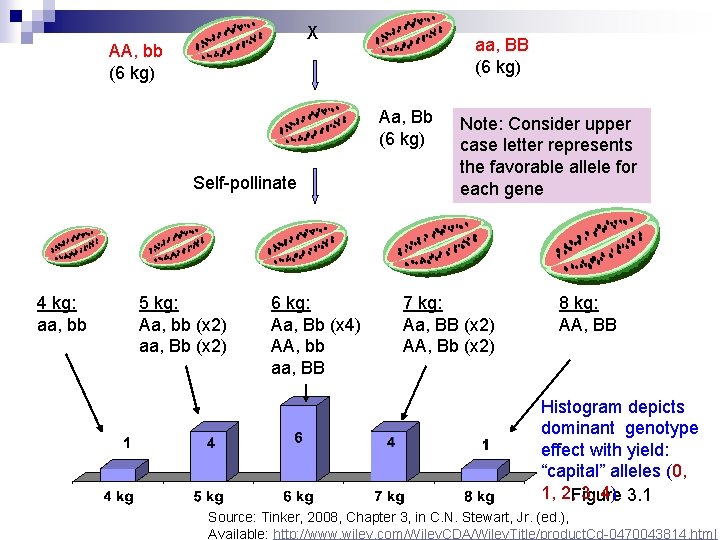
X AA, bb (6 kg) aa, BB (6 kg) Aa, Bb (6 kg) Self-pollinate 4 kg: aa, bb 5 kg: Aa, bb (x 2) aa, Bb (x 2) 6 kg: Aa, Bb (x 4) AA, bb aa, BB Note: Consider upper case letter represents the favorable allele for each gene 7 kg: Aa, BB (x 2) AA, Bb (x 2) 8 kg: AA, BB Histogram depicts dominant genotype effect with yield: “capital” alleles (0, 1, 2, Figure 3, 4) 3. 1 Source: Tinker, 2008, Chapter 3, in C. N. Stewart, Jr. (ed. ), Available: http: //www. wiley. com/Wiley. CDA/Wiley. Title/product. Cd-0470043814. html
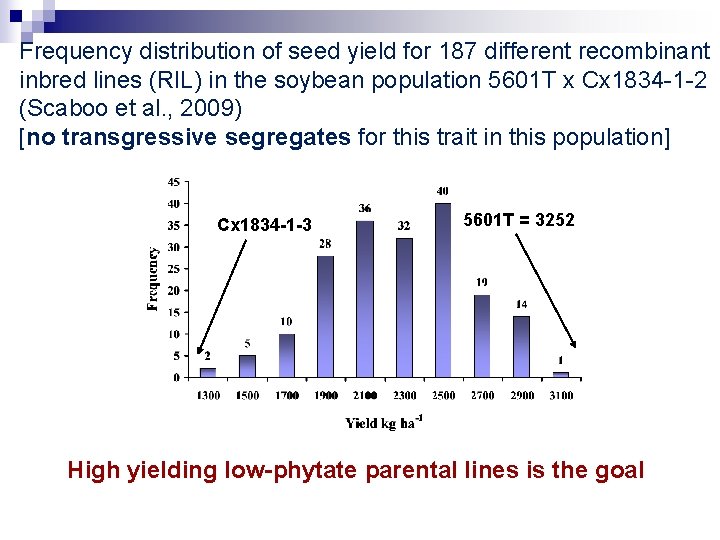
Frequency distribution of seed yield for 187 different recombinant inbred lines (RIL) in the soybean population 5601 T x Cx 1834 -1 -2 (Scaboo et al. , 2009) [no transgressive segregates for this trait in this population] Cx 1834 -1 -3 5601 T = 3252 High yielding low-phytate parental lines is the goal
![= [1 -(½)G]L Adapted from Allard, 1999 15/16 7/8 3/4 1/2 (15/16)20 Then find = [1 -(½)G]L Adapted from Allard, 1999 15/16 7/8 3/4 1/2 (15/16)20 Then find](http://slidetodoc.com/presentation_image_h2/faaeff577b81b61ab5836e6919c095e5/image-43.jpg)
= [1 -(½)G]L Adapted from Allard, 1999 15/16 7/8 3/4 1/2 (15/16)20 Then find the better individuals among the homozygous plants (those accumulating the greatest number of superior alleles). Can be done with DNA technologies and progeny row testing. (7/8)20 (1/2)20 (3/4)20 Even if 20 genes is involved, using the power of inbreeding 5 generations, over half the proportion of individuals will be completely homozygous!

How do we breed improved crop cultivars? 2. Understand the effect of reproductive behavior
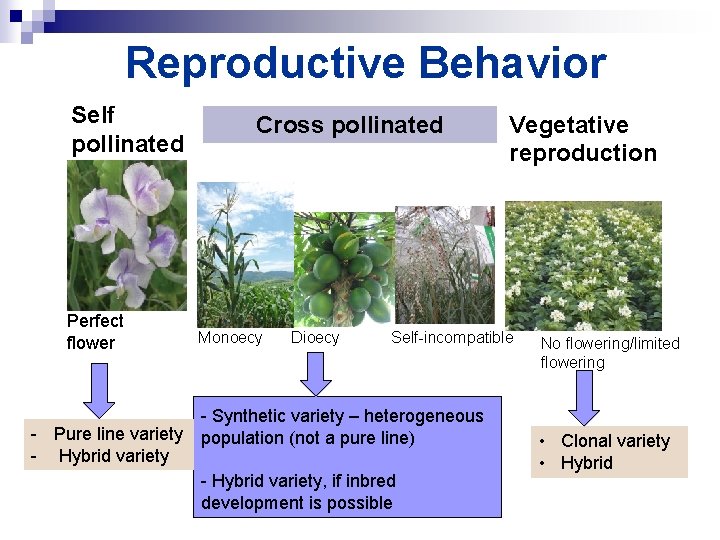
Reproductive Behavior Self pollinated Perfect flower - Pure line variety - Hybrid variety Cross pollinated Monoecy Dioecy Vegetative reproduction Self-incompatible - Synthetic variety – heterogeneous population (not a pure line) - Hybrid variety, if inbred development is possible No flowering/limited flowering • Clonal variety • Hybrid
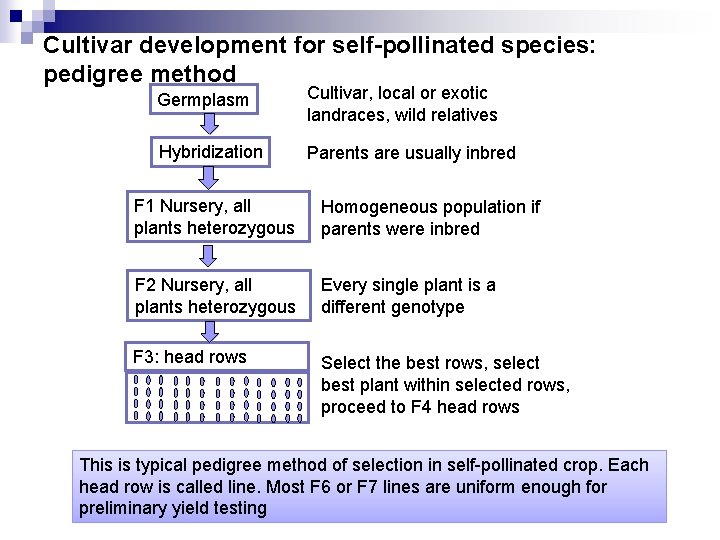
Cultivar development for self-pollinated species: pedigree method Germplasm Cultivar, local or exotic landraces, wild relatives Hybridization Parents are usually inbred F 1 Nursery, all plants heterozygous Homogeneous population if parents were inbred F 2 Nursery, all plants heterozygous Every single plant is a different genotype F 3: head rows Select the best rows, select best plant within selected rows, proceed to F 4 head rows This is typical pedigree method of selection in self-pollinated crop. Each head row is called line. Most F 6 or F 7 lines are uniform enough for preliminary yield testing
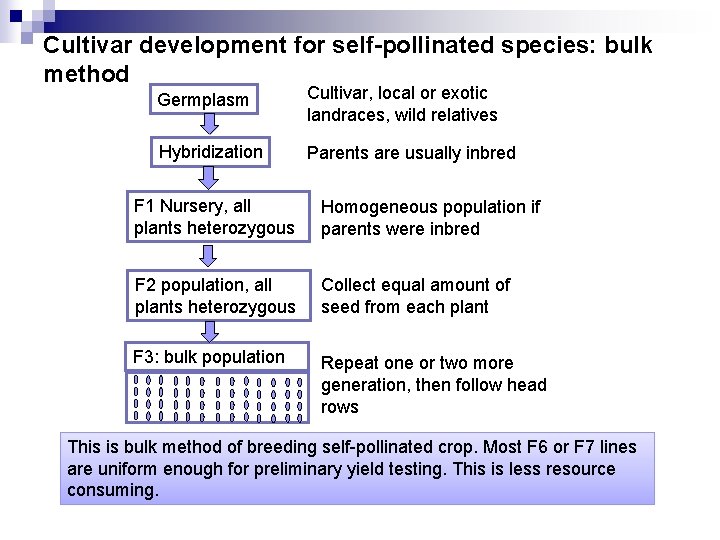
Cultivar development for self-pollinated species: bulk method Germplasm Cultivar, local or exotic landraces, wild relatives Hybridization Parents are usually inbred F 1 Nursery, all plants heterozygous Homogeneous population if parents were inbred F 2 population, all plants heterozygous Collect equal amount of seed from each plant F 3: bulk population Repeat one or two more generation, then follow head rows This is bulk method of breeding self-pollinated crop. Most F 6 or F 7 lines are uniform enough for preliminary yield testing. This is less resource consuming.
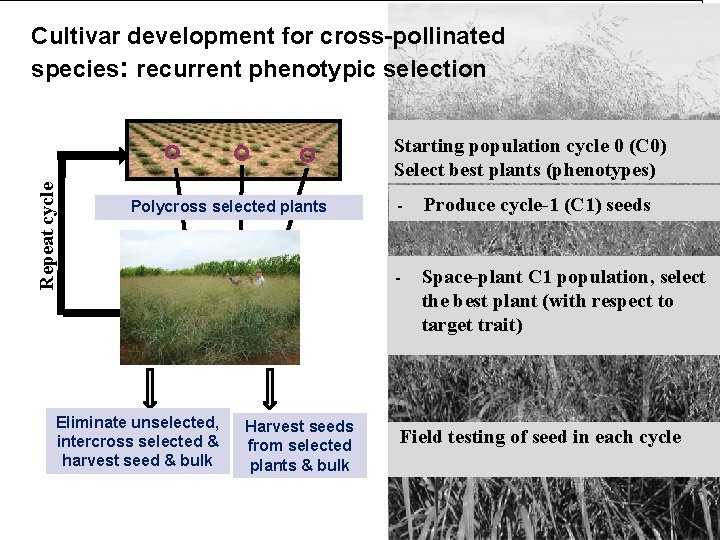
Cultivar development for cross-pollinated species: recurrent phenotypic selection Repeat cycle Starting population cycle 0 (C 0) Select best plants (phenotypes) Polycross selected plants Eliminate unselected, intercross selected & harvest seed & bulk Harvest seeds from selected plants & bulk - Produce cycle-1 (C 1) seeds - Space-plant C 1 population, select the best plant (with respect to target trait) Field testing of seed in each cycle
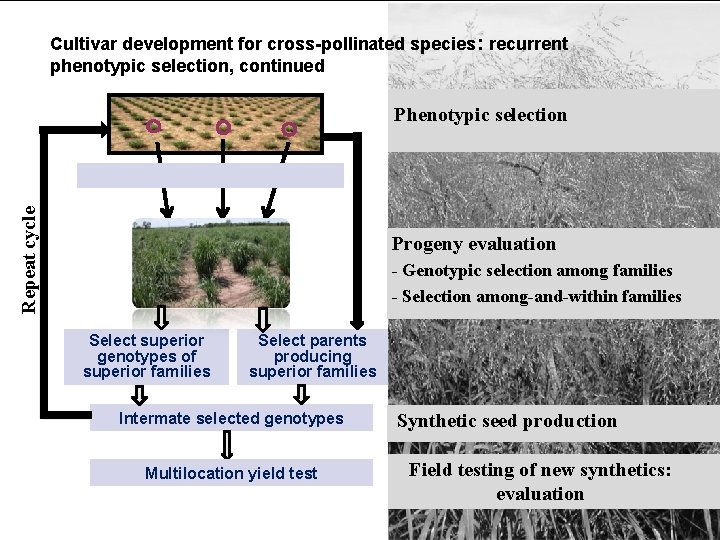
Cultivar development for cross-pollinated species: recurrent phenotypic selection, continued Repeat cycle Phenotypic selection Progeny evaluation - Genotypic selection among families - Selection among-and-within families Select superior genotypes of superior families Select parents producing superior families Intermate selected genotypes Multilocation yield test Synthetic seed production Field testing of new synthetics: evaluation

How cultivar development can be accelerated One method: backcross breeding
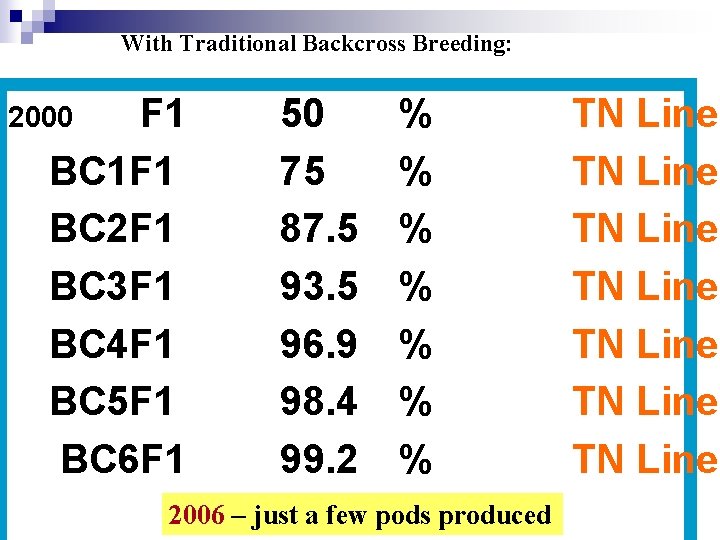
With Traditional Backcross Breeding: F 1 BC 1 F 1 BC 2 F 1 BC 3 F 1 BC 4 F 1 BC 5 F 1 BC 6 F 1 2000 50 75 87. 5 93. 5 96. 9 98. 4 99. 2 % % % % 2006 – just a few pods produced TN Line TN Line
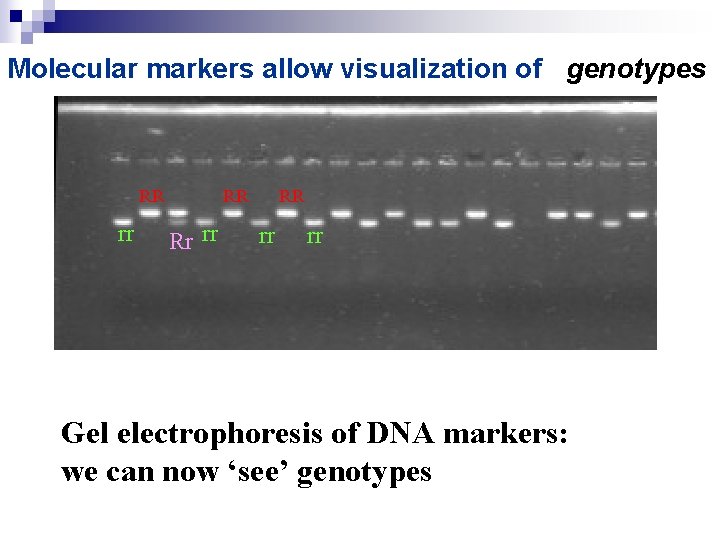
Molecular markers allow visualization of genotypes RR rr RR Rr rr RR rr rr Gel electrophoresis of DNA markers: we can now ‘see’ genotypes
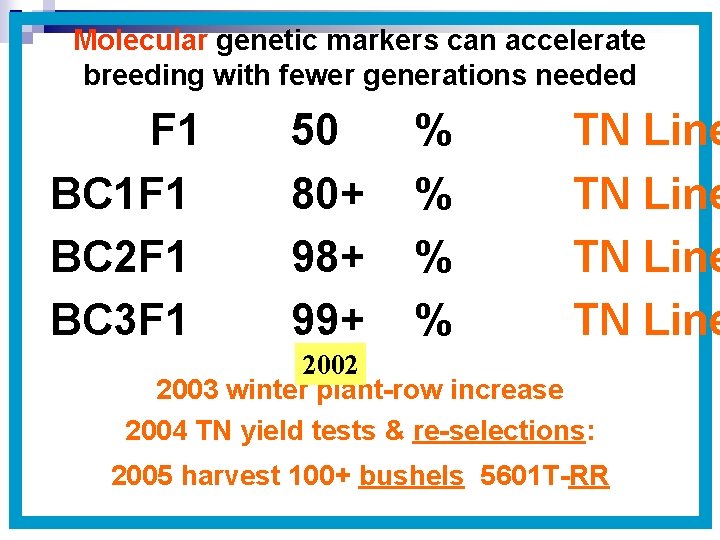
Molecular genetic markers can accelerate breeding with fewer generations needed F 1 BC 1 F 1 BC 2 F 1 BC 3 F 1 50 80+ 98+ 99+ % % TN Line 2002 2003 winter plant-row increase 2004 TN yield tests & re-selections: 2005 harvest 100+ bushels 5601 T-RR
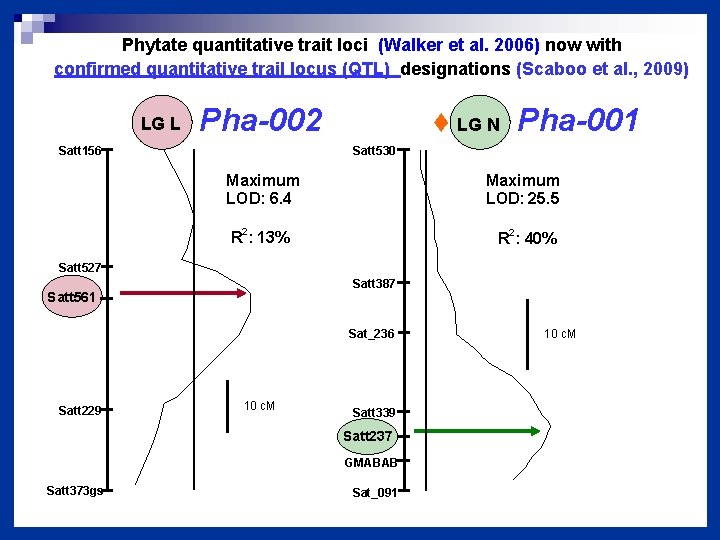
Phytate quantitative trait loci (Walker et al. 2006) now with confirmed quantitative trail locus (QTL) designations (Scaboo et al. , 2009) LG L Pha-002 Satt 156 ♦ LG N Pha-001 Satt 530 Maximum LOD: 6. 4 Maximum LOD: 25. 5 2 2 R : 13% R : 40% Satt 527 Satt 387 Satt 561 Sat_236 Satt 229 10 c. M Satt 339 Satt 237 GMABAB Satt 373 gs Sat_091 10 c. M
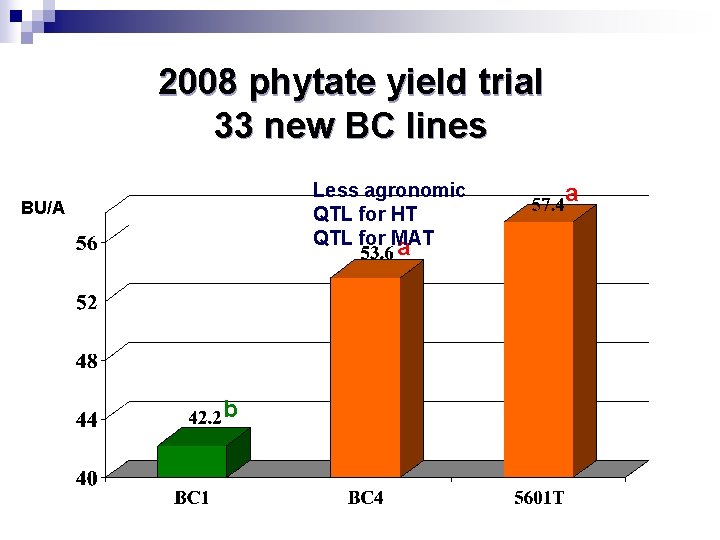
2008 phytate yield trial 33 new BC lines Less agronomic QTL for HT QTL for MAT a BU/A b a

Biotechnology can be used to improve crop cultivars? 3. Transgenic varieties
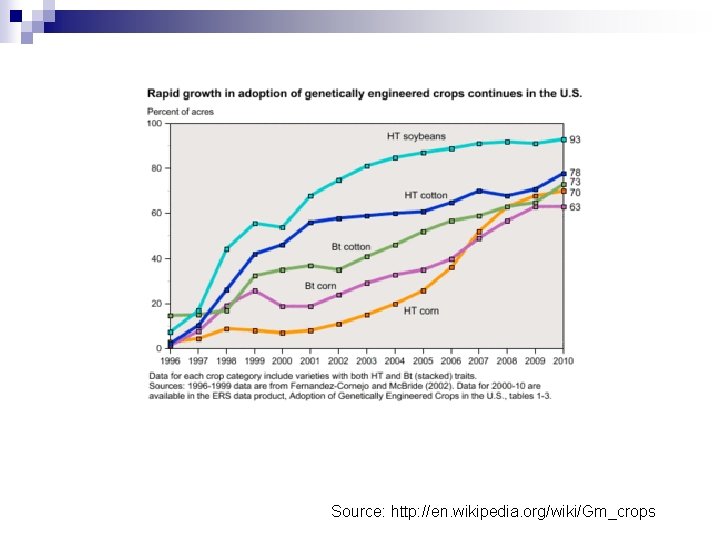
Source: http: //en. wikipedia. org/wiki/Gm_crops
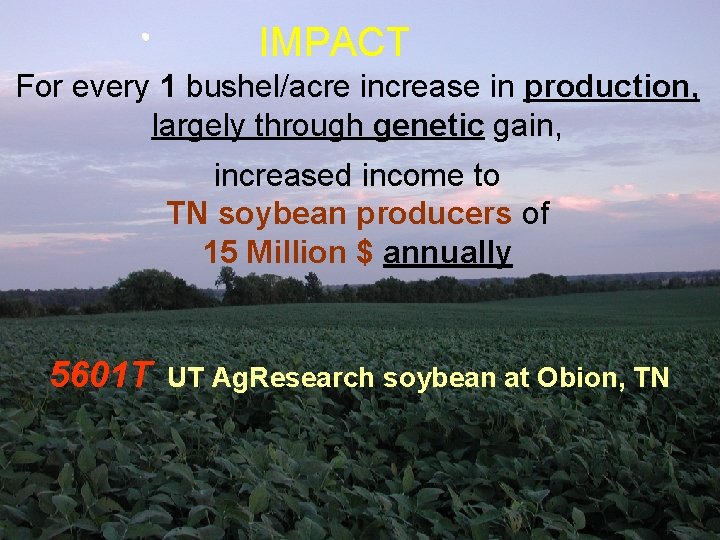
IMPACT For every 1 bushel/acre increase in production, largely through genetic gain, increased income to TN soybean producers of 15 Million $ annually 5601 T UT Ag. Research soybean at Obion, TN
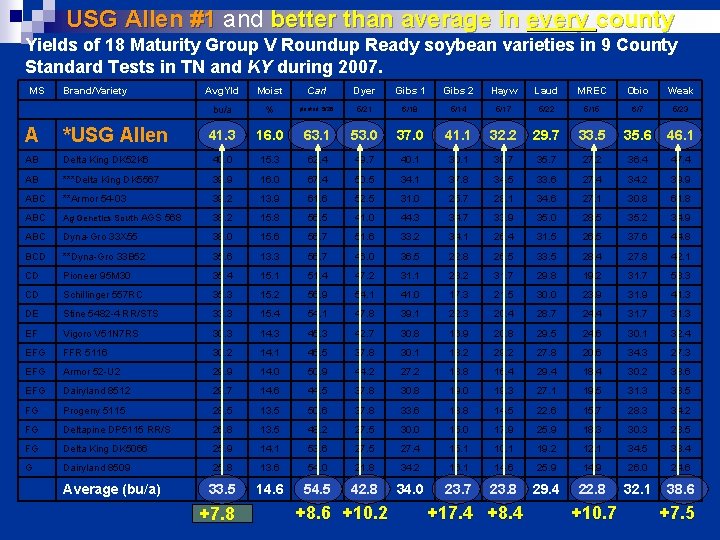
USG Allen #1 and better than average in every county Yields of 18 Maturity Group V Roundup Ready soybean varieties in 9 County Standard Tests in TN and KY during 2007. MS Brand/Variety Avg. Yld Moist Carl Dyer Gibs 1 Gibs 2 Hayw Laud MREC Obio Weak bu/a % planted 5/26 5/21 6/18 5/14 5/17 5/22 5/15 6/7 5/23 41. 3 16. 0 63. 1 53. 0 37. 0 41. 1 32. 2 29. 7 33. 5 35. 6 46. 1 A *USG Allen AB Delta King DK 52 K 6 40. 0 15. 3 62. 4 49. 7 40. 1 30. 7 35. 7 27. 2 36. 4 47. 4 AB ***Delta King DK 5567 39. 9 16. 0 67. 4 50. 5 34. 1 37. 8 34. 5 33. 6 27. 4 34. 2 39. 9 ABC **Armor 54 -03 39. 2 13. 9 61. 6 52. 5 31. 0 25. 7 28. 1 34. 6 27. 1 30. 8 61. 8 ABC Ag Genetics South AGS 568 38. 2 15. 8 56. 5 41. 0 44. 3 34. 7 33. 9 35. 0 28. 5 35. 2 34. 9 ABC Dyna-Gro 33 X 55 38. 0 15. 6 56. 7 51. 6 33. 2 34. 1 26. 4 31. 5 26. 5 37. 6 44. 8 BCD **Dyna-Gro 33 B 52 35. 6 13. 3 56. 7 46. 0 36. 5 22. 8 26. 5 33. 5 28. 4 27. 8 42. 1 CD Pioneer 95 M 30 35. 4 15. 1 51. 4 47. 2 31. 1 23. 2 31. 7 29. 8 19. 2 31. 7 53. 3 CD Schillinger 557 RC 35. 3 15. 2 56. 9 54. 1 41. 0 17. 3 21. 5 30. 0 23. 9 31. 9 41. 3 DE Stine 5482 -4 RR/STS 33. 3 15. 4 54. 1 47. 8 39. 1 22. 3 20. 4 28. 7 24. 4 31. 7 31. 3 EF Vigoro V 51 N 7 RS 30. 3 14. 3 45. 3 42. 7 30. 8 16. 9 20. 8 29. 5 24. 6 30. 1 32. 4 EFG FFR 5116 30. 2 14. 1 46. 5 37. 8 30. 1 18. 2 29. 2 27. 8 20. 6 34. 3 27. 3 EFG Armor 52 -U 2 29. 9 14. 0 50. 9 44. 2 27. 2 18. 8 16. 4 29. 4 18. 4 30. 2 33. 6 EFG Dairyland 8512 29. 7 14. 6 44. 5 37. 8 30. 8 19. 0 19. 3 27. 1 19. 5 31. 3 38. 5 FG Progeny 5115 28. 5 13. 5 50. 6 37. 8 33. 6 18. 8 14. 5 22. 6 15. 7 28. 3 34. 2 FG Deltapine DP 5115 RR/S 26. 8 13. 5 48. 2 27. 5 30. 0 15. 0 17. 9 25. 9 18. 3 30. 3 28. 5 FG Delta King DK 5066 25. 9 14. 1 53. 6 27. 5 27. 4 15. 1 10. 1 19. 2 12. 1 34. 5 33. 4 G Dairyland 8509 25. 8 13. 6 54. 0 21. 8 34. 2 16. 1 14. 6 25. 9 14. 9 26. 0 24. 6 33. 5 14. 6 54. 5 42. 8 34. 0 23. 7 23. 8 29. 4 22. 8 32. 1 38. 6 Average (bu/a) +7. 8 +8. 6 +10. 2 +17. 4 +8. 4 +10. 7 +7. 5

For the plant breeder patience is a virtue …when working with new genetics

Key points Know basic terminology in transmission genetics and plant breeding n Understand the goals of plant breeding n Know plant reproductive syndromes, e. g. , self-fertilization, and how they effect breeding methods n
- Slides: 61