Placenta Research Network Meeting Journal Club Naveen K

Placenta Research Network Meeting Journal Club Naveen K. Neradugomma 04/29/2016


Placenta • Transient organ of fetal origin, develops during pregnancy • A critical organ for a successful pregnancy • Hormone production • Nutrient and waste exchange • Limit infection/immunity responses • Limits xenobiotic exposure • Defects lead to pregnancy complications and/or fetal growth defects
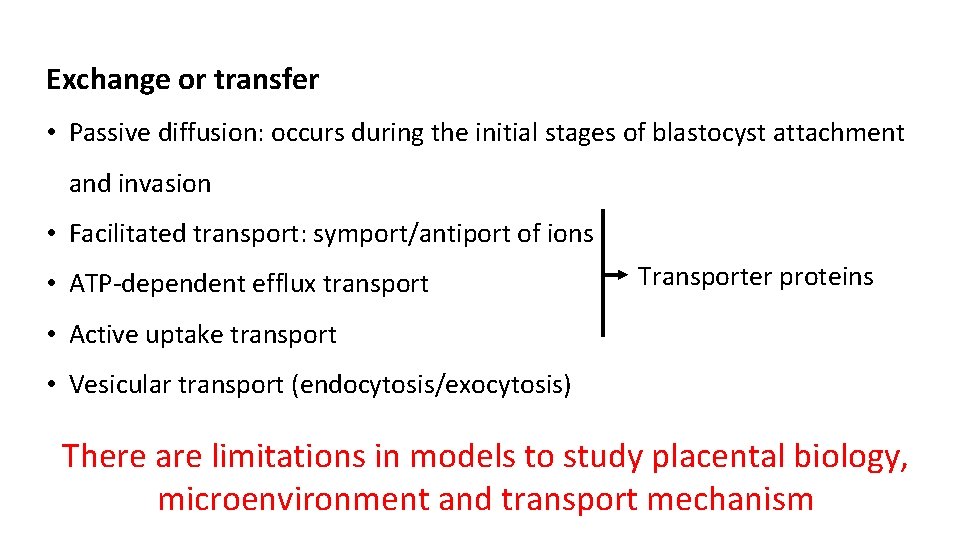
Exchange or transfer • Passive diffusion: occurs during the initial stages of blastocyst attachment and invasion • Facilitated transport: symport/antiport of ions • ATP-dependent efflux transport Transporter proteins • Active uptake transport • Vesicular transport (endocytosis/exocytosis) There are limitations in models to study placental biology, microenvironment and transport mechanism
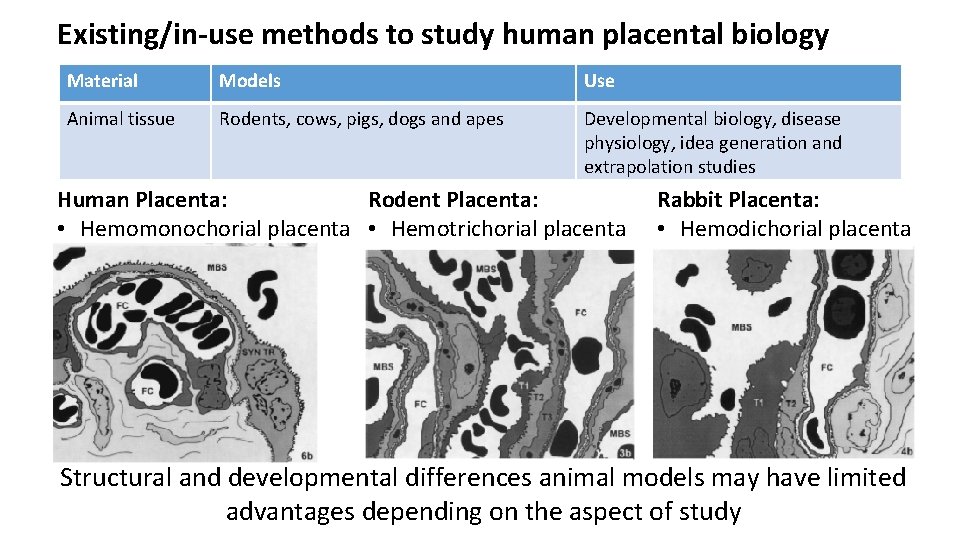
Existing/in-use methods to study human placental biology Material Models Use Animal tissue Rodents, cows, pigs, dogs and apes Developmental biology, disease physiology, idea generation and extrapolation studies Whole tissue perfusion Trans-placental xenobiotic Human Placenta: Ex vivo human placenta Rodent Placenta: Rabbittransfer, Placenta: metabolism and transporter function • Hemomonochorial placenta • Hemotrichorial placenta • Hemodichorial placenta Tissue pieces Explant culture Villous explant cultures Transfer, uptake, transport and metabolism Cells Primary trophoblast cells Choriocarcinoma cells Cell biology, transfer, transport, metabolism Tissue fractions Microsomes, cytoplasmic fraction, microvillus and basal membrane vesicles Xenobiotic metabolism, transport function. Humans Pregnant females Phase trials Structural and developmental differences animal models may have limited advantages depending on the aspect of study
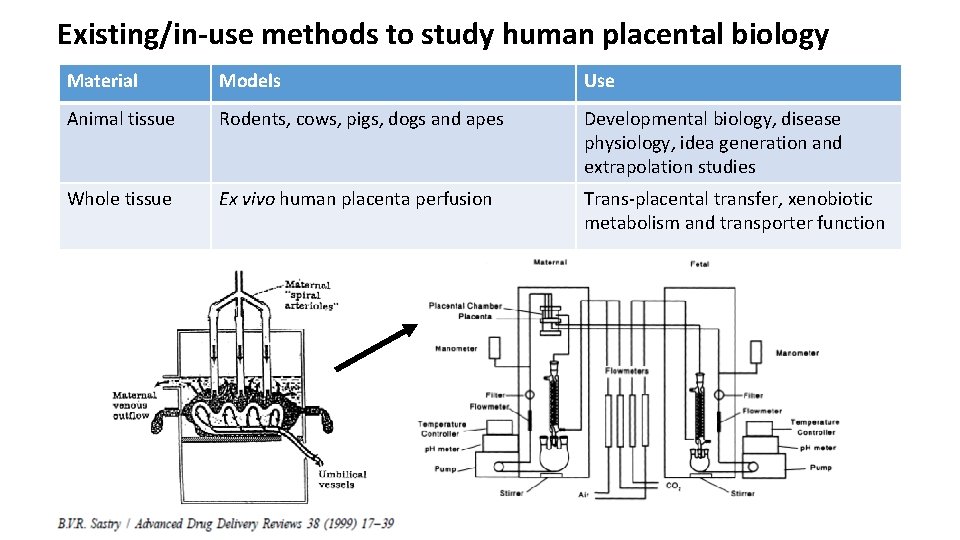
Existing/in-use methods to study human placental biology Material Models Use Animal tissue Rodents, cows, pigs, dogs and apes Developmental biology, disease physiology, idea generation and extrapolation studies Whole tissue Ex vivo human placenta perfusion Trans-placental transfer, xenobiotic metabolism and transporter function Tissue pieces Explant culture Villous explant cultures Transfer, uptake, transport and metabolism Cells Primary trophoblast cells Choriocarcinoma cells Cell biology, transfer, transport, metabolism Tissue fractions Microsomes, cytoplasmic fraction, microvillus and basal membrane vesicles Xenobiotic metabolism, transport function. Humans Pregnant females Phase trials
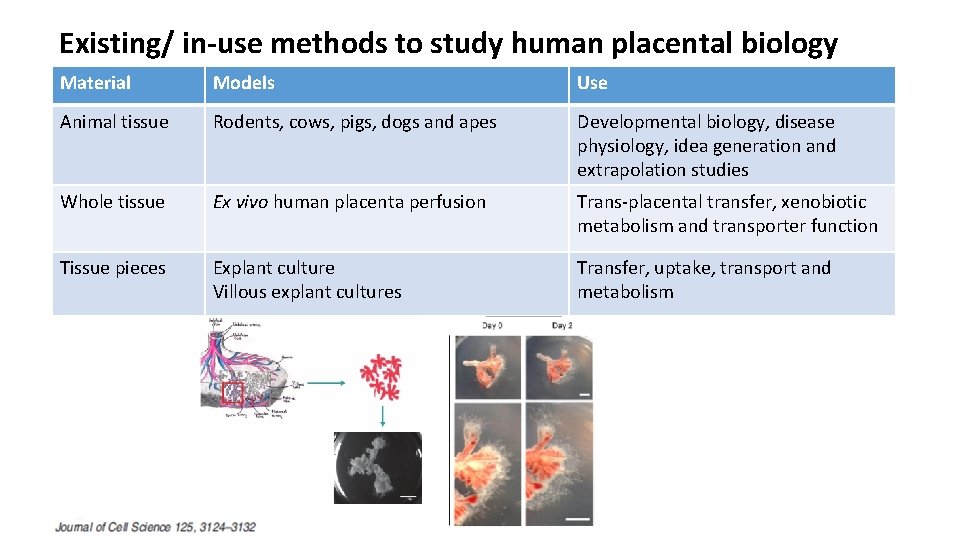
Existing/ in-use methods to study human placental biology Material Models Use Animal tissue Rodents, cows, pigs, dogs and apes Developmental biology, disease physiology, idea generation and extrapolation studies Whole tissue Ex vivo human placenta perfusion Trans-placental transfer, xenobiotic metabolism and transporter function Tissue pieces Explant culture Villous explant cultures Transfer, uptake, transport and metabolism Cells Primary trophoblast cells Choriocarcinoma cells Cell biology, transfer, transport, metabolism Tissue fractions Microsomes, cytoplasmic fraction, microvillus and basal membrane vesicles Xenobiotic metabolism, transport function. Humans Pregnant females Phase trials
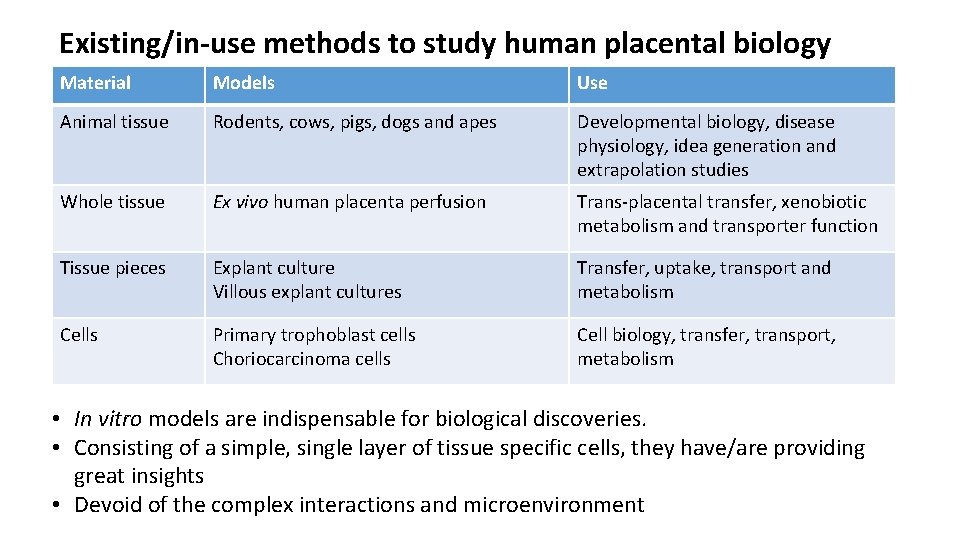
Existing/in-use methods to study human placental biology Material Models Use Animal tissue Rodents, cows, pigs, dogs and apes Developmental biology, disease physiology, idea generation and extrapolation studies Whole tissue Ex vivo human placenta perfusion Trans-placental transfer, xenobiotic metabolism and transporter function Tissue pieces Explant culture Villous explant cultures Transfer, uptake, transport and metabolism Cells Primary trophoblast cells Choriocarcinoma cells Cell biology, transfer, transport, metabolism Tissue fractions Microsomes, cytoplasmic fraction, Xenobiotic metabolism, transport In vitro modelsmicrovillus are indispensable for biological and basal membrane vesiclesdiscoveries. function. • • Humans Consisting of a. Pregnant simple, females single layer of tissue specific Drug cells, trialsthey. Difficult have/are providing to get approval/ great insights consent and/or numbers • Devoid of the complex interactions and microenvironment
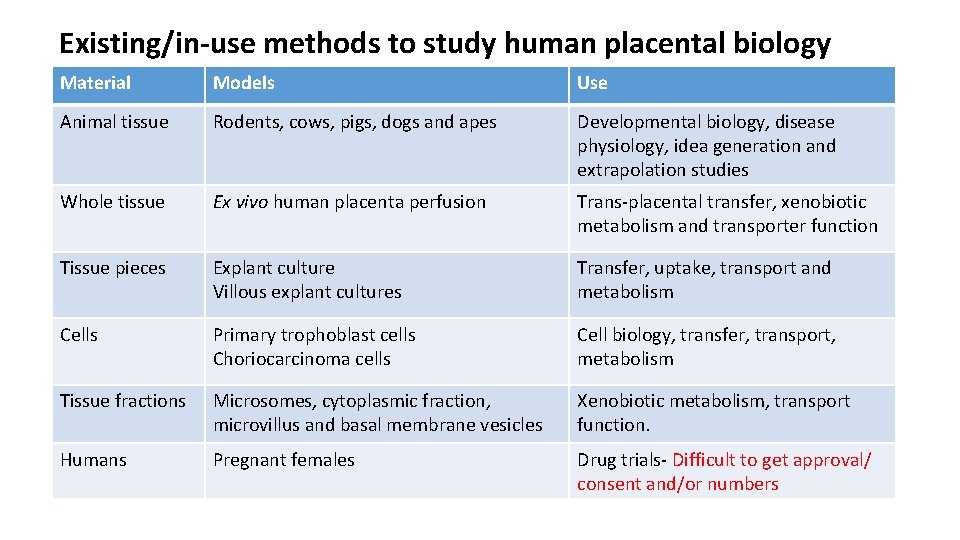
Existing/in-use methods to study human placental biology Material Models Use Animal tissue Rodents, cows, pigs, dogs and apes Developmental biology, disease physiology, idea generation and extrapolation studies Whole tissue Ex vivo human placenta perfusion Trans-placental transfer, xenobiotic metabolism and transporter function Tissue pieces Explant culture Villous explant cultures Transfer, uptake, transport and metabolism Cells Primary trophoblast cells Choriocarcinoma cells Cell biology, transfer, transport, metabolism Tissue fractions Microsomes, cytoplasmic fraction, microvillus and basal membrane vesicles Xenobiotic metabolism, transport function. Humans Pregnant females Drug trials- Difficult to get approval/ consent and/or numbers

Organ-on-a-chip/Micro-physiology/ micro-engineered organ model

Organ-on-a-chip A combination of cell culture (single or multiple) and fluid/media flow, all on a chip, to simulate the activities, mechanics and physiological response of an entire organ and organ systems.
Organ-on-a-chip UW/Nortis System: Thomas Neumann and Alan Nelson
Micro-engineered organs Yum K et, al: Biotechnol. J. , 9, 16 -27, 2014.
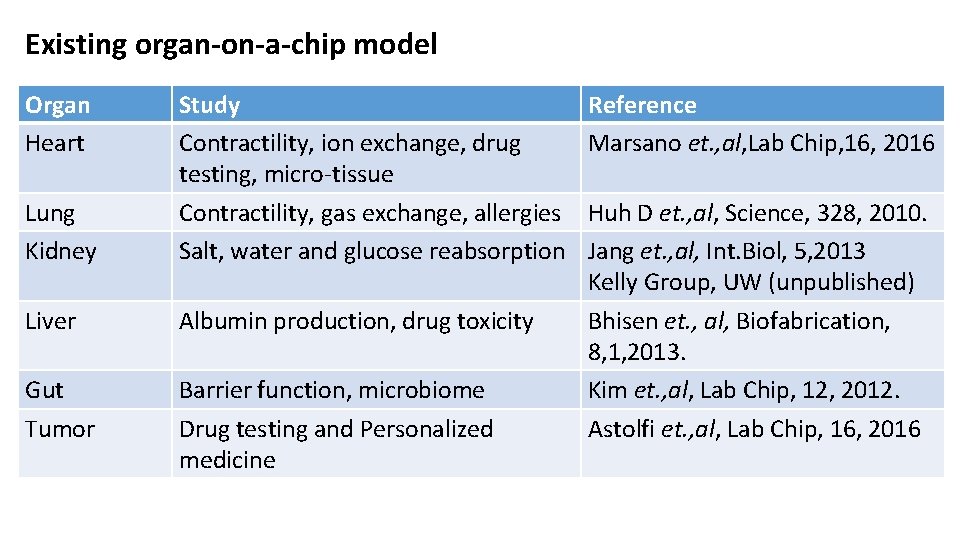
Existing organ-on-a-chip model Organ Heart Lung Kidney Study Contractility, ion exchange, drug testing, micro-tissue Contractility, gas exchange, allergies Salt, water and glucose reabsorption Liver Albumin production, drug toxicity Gut Tumor Barrier function, microbiome Drug testing and Personalized medicine Reference Marsano et. , al, Lab Chip, 16, 2016 Huh D et. , al, Science, 328, 2010. Jang et. , al, Int. Biol, 5, 2013 Kelly Group, UW (unpublished) Bhisen et. , al, Biofabrication, 8, 1, 2013. Kim et. , al, Lab Chip, 12, 2012. Astolfi et. , al, Lab Chip, 16, 2016
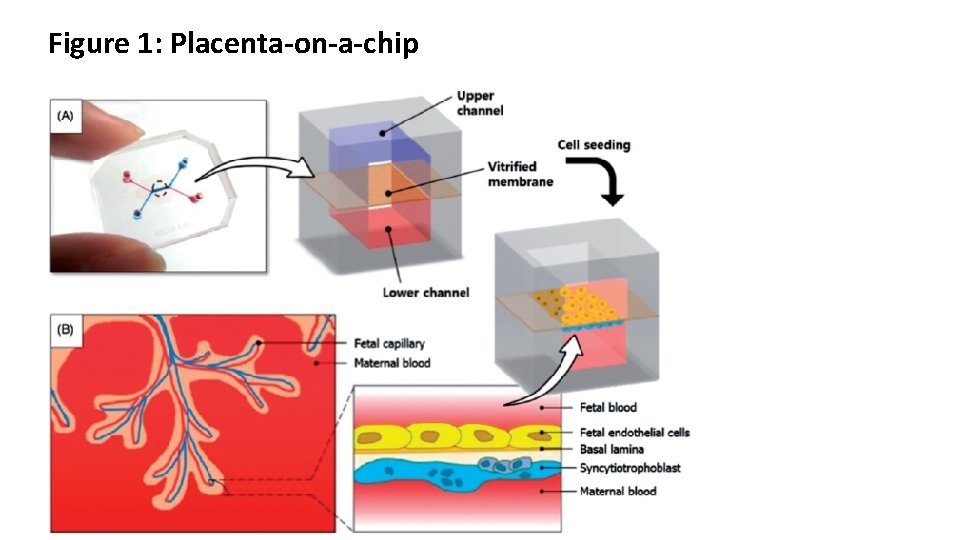
Figure 1: Placenta-on-a-chip
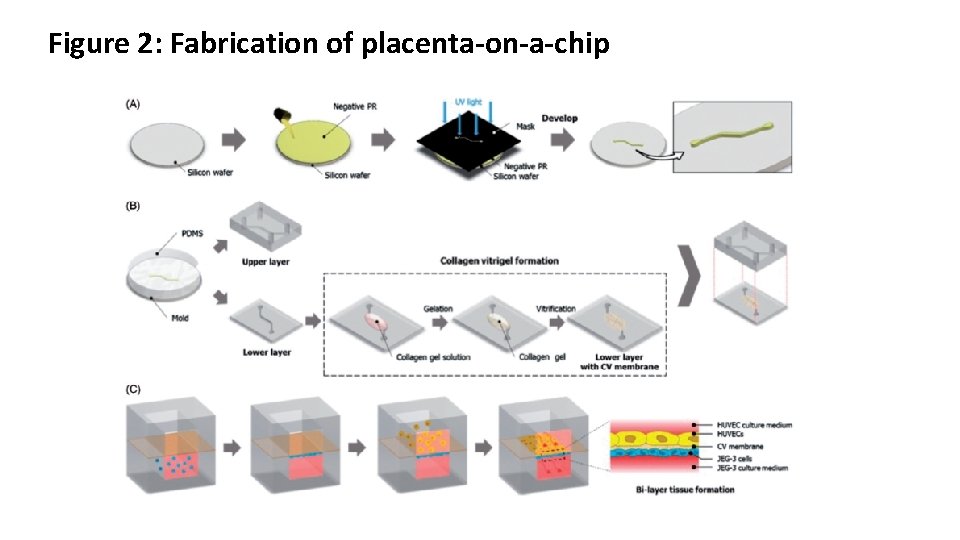
Figure 2: Fabrication of placenta-on-a-chip
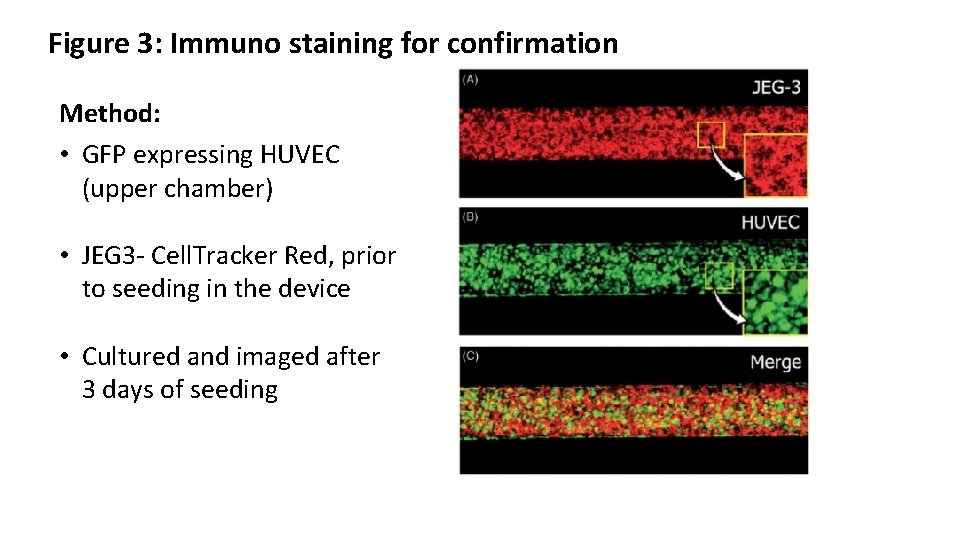
Figure 3: Immuno staining for confirmation Method: • GFP expressing HUVEC (upper chamber) • JEG 3 - Cell. Tracker Red, prior to seeding in the device • Cultured and imaged after 3 days of seeding
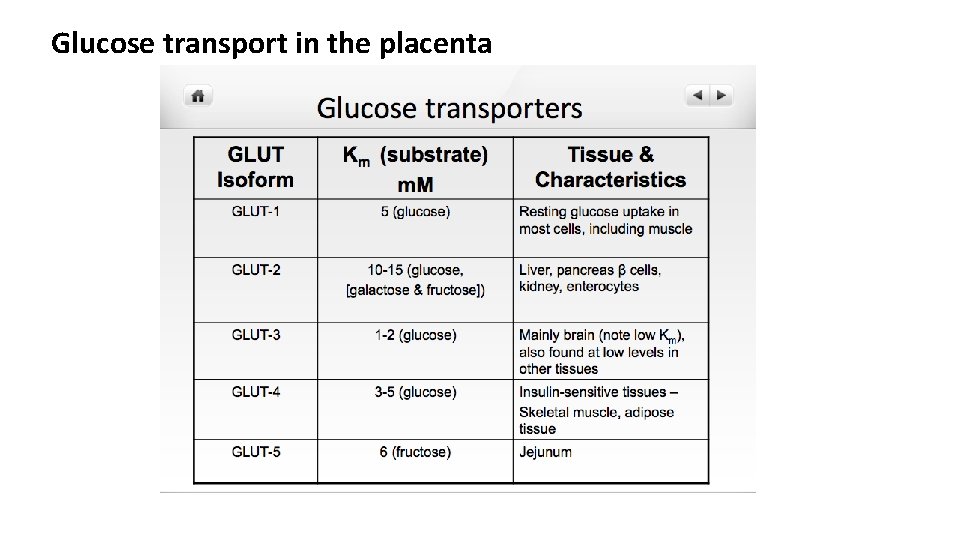
Glucose transport in the placenta
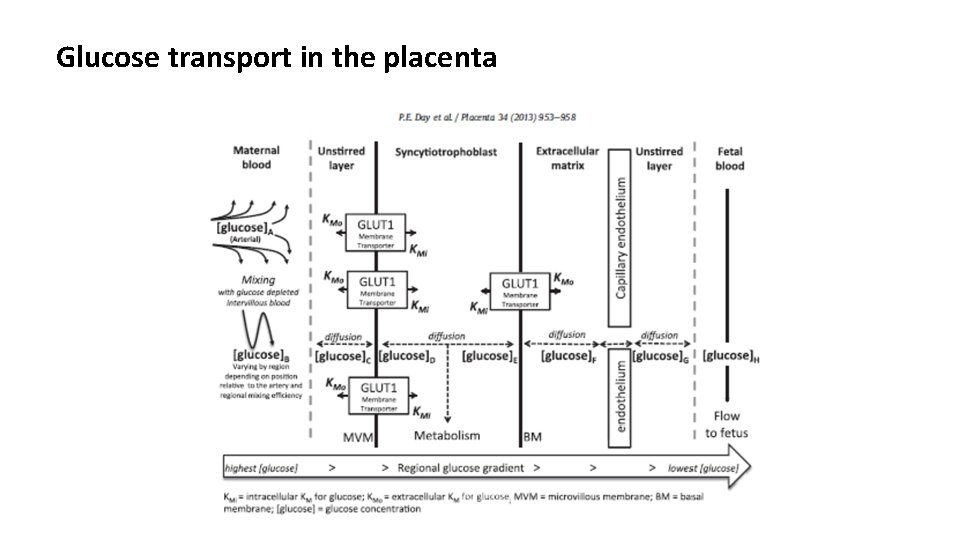
Glucose transport in the placenta
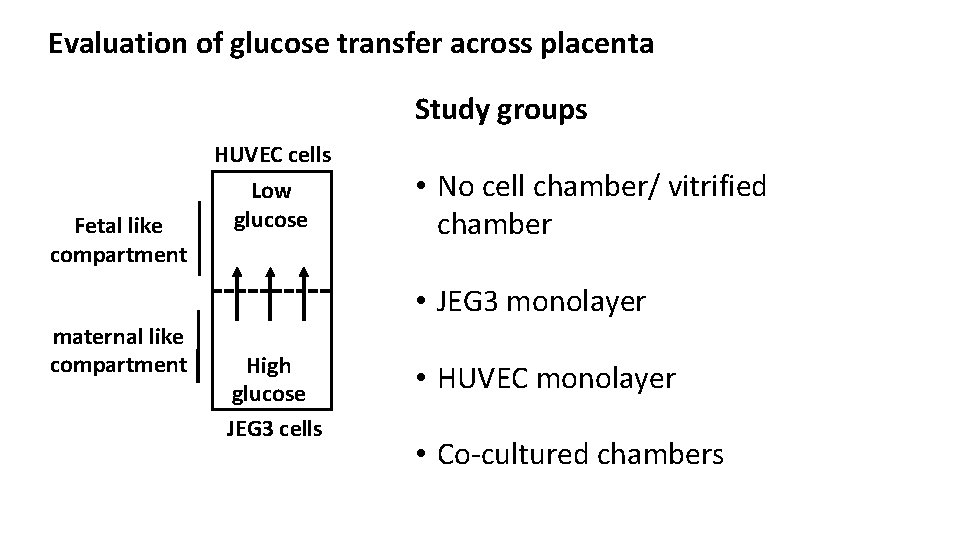
Evaluation of glucose transfer across placenta Study groups HUVEC cells Fetal like compartment maternal like compartment Low glucose • No cell chamber/ vitrified chamber High glucose • JEG 3 monolayer High glucose JEG 3 cells • HUVEC monolayer • Co-cultured chambers
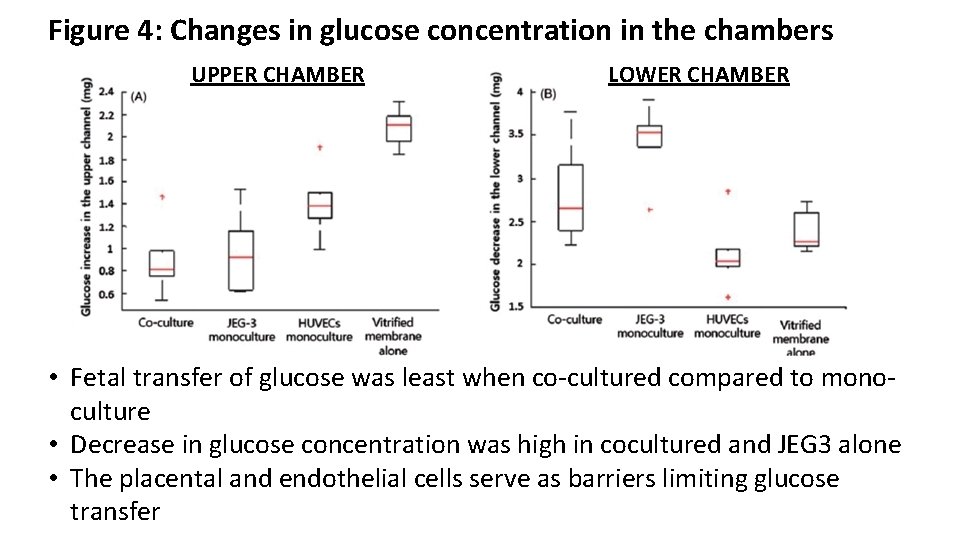
Figure 4: Changes in glucose concentration in the chambers UPPER CHAMBER LOWER CHAMBER • Fetal transfer of glucose was least when co-cultured compared to monoculture • Decrease in glucose concentration was high in cocultured and JEG 3 alone • The placental and endothelial cells serve as barriers limiting glucose transfer
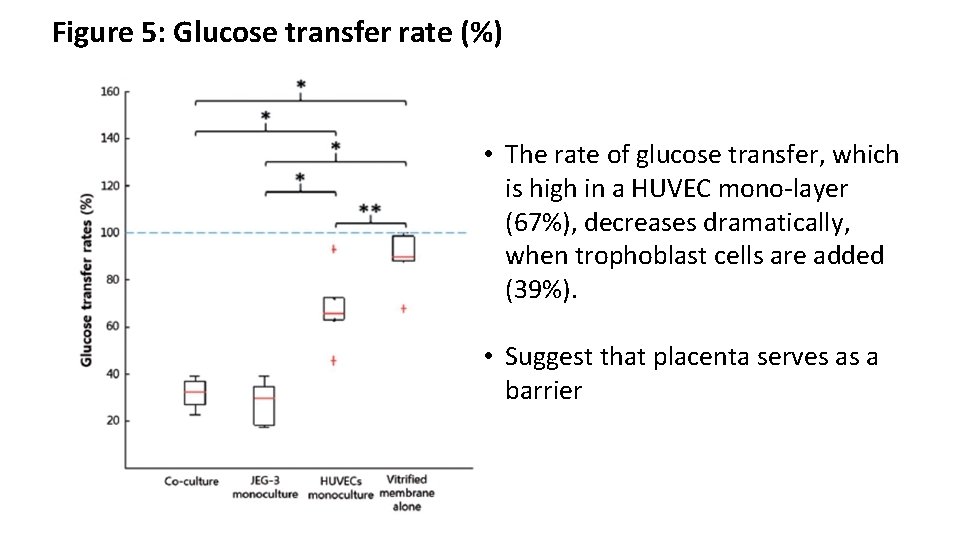
Figure 5: Glucose transfer rate (%) • The rate of glucose transfer, which is high in a HUVEC mono-layer (67%), decreases dramatically, when trophoblast cells are added (39%). • Suggest that placenta serves as a barrier
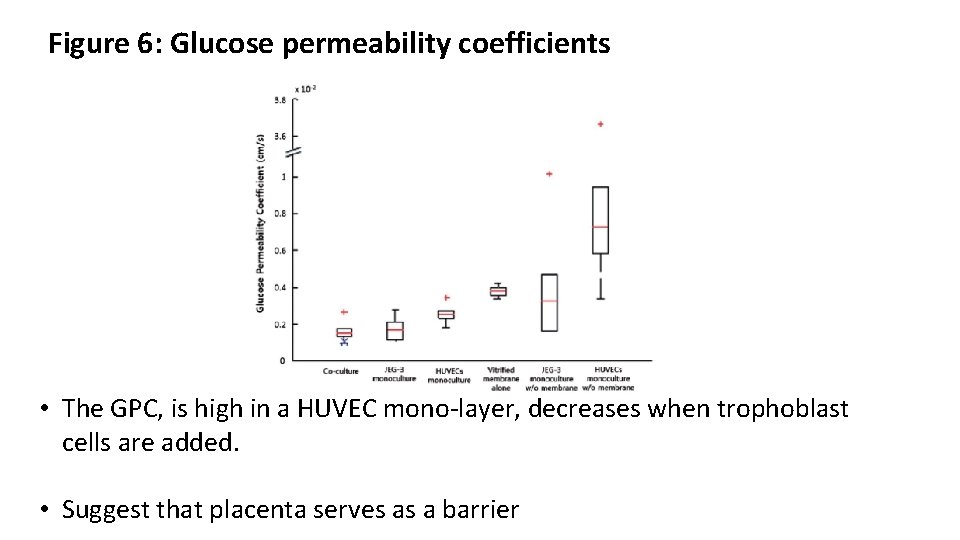
Figure 6: Glucose permeability coefficients • The GPC, is high in a HUVEC mono-layer, decreases when trophoblast cells are added. • Suggest that placenta serves as a barrier
Overall conclusions • Established placental microphysiology system • Suggest that placenta serves as a barrier, limiting glucose transfer to fetus • Provides a novel platform for placental physiology studies and tissue interactions Shortfalls • JEG 3 cells- use induced differentiation model • Be. Wo cells- use induced differentiation model • HUVEC- vein endothelial cells- limited expression of GLUT’s

Future plans of our team • Use primary human villous trophoblast cells • Work on single cell and multiple cell model • Placental exosomes and tissue interaction
- Slides: 25