Pitlochry Primary School Online Programme 1 Dear Grade






















- Slides: 33

Pitlochry Primary School Online Programme 1

Dear Grade 6 Scientists This week, we are learning about Water Purification. Water is essential for all life on Earth. It is important for us to preserve the quality of the water on Earth. Please click play on the audio links as you work through this task. I trust that you have completed the activity that was uploaded last week. Enjoy the following lesson. Remember to always try your best. Have fun and I hope to see you all soon! With love, Mrs N Chetty 2

Lesson 13 Water Purification Grade: 6 Presented By: Mrs N Chetty Pitlochry Primary School Educator 3

Living things such as plants and animals rely on water. Life cannot exist without water. The importance of clean water Water management is everyone’s responsibility but the municipality officials of towns and cities have the responsibility of looking after the water treatment facilities. The municipality must purify water to keep people healthy. 4

Purifying Water There are four ways to purify water • Boiling • Filtering • Settling and Decanting • Chemical Treatment 5

1. Boiling Boiled water is heated to kill any germs in the water. This method uses a lot of electricity or firewood and does not remove soluble or insoluble pollution. 6

2. Filtering Pouring water through a funnel and filter paper removes most of the insoluble pollution such as sand but germs can pass through the filter paper. Soluble substances such as chemicals, fertilisers and pesticides are also not removed. 7

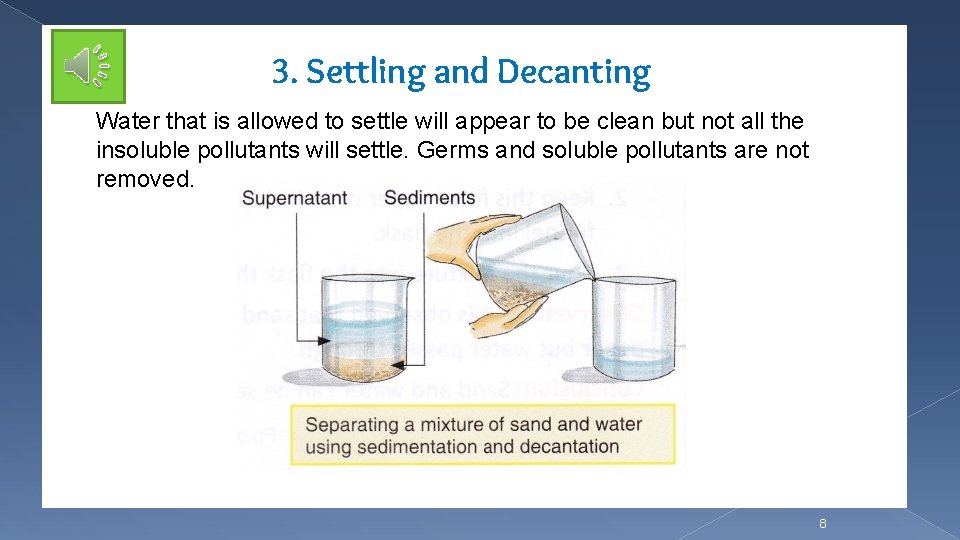
3. Settling and Decanting Water that is allowed to settle will appear to be clean but not all the insoluble pollutants will settle. Germs and soluble pollutants are not removed. 8

4. Chemical Treatment Chemicals can be added to water to kill germs but these chemicals are expensive and can make the water taste awful. 9

Activity 13 Draw a table that compares the advantages and disadvantages of each method of water purification listed above. Purification Method Advantages Disadvantages Boiling Filtering Settling and Decanting Chemical treatment 10

The Story behind tap water The Source A dam is built across a river, water from the dam is pumped to the Water Purification Station. 11 11

1. Screening At the purification station water passes through metal screens to trap large living organisms, sticks, leaves and litter, but allows the rest of the water to pass through it. 12 12

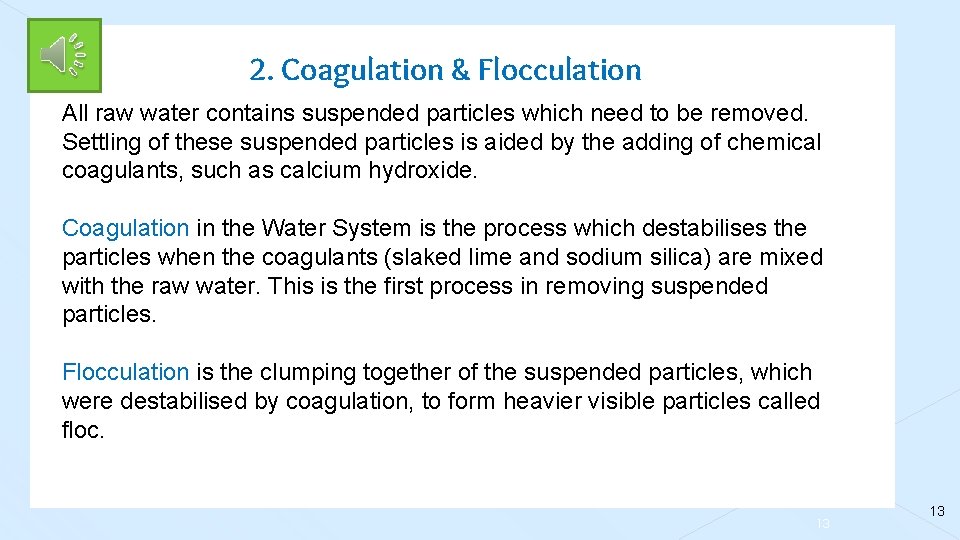
2. Coagulation & Flocculation All raw water contains suspended particles which need to be removed. Settling of these suspended particles is aided by the adding of chemical coagulants, such as calcium hydroxide. Coagulation in the Water System is the process which destabilises the particles when the coagulants (slaked lime and sodium silica) are mixed with the raw water. This is the first process in removing suspended particles. Flocculation is the clumping together of the suspended particles, which were destabilised by coagulation, to form heavier visible particles called floc. 13 13

3. Sedimentation The water flows slowly into large sedimentation tanks. The floc then settles to the bottom of the tank to form sludge. 14 14

4. Carbonation After sedimentation, the water flows into carbonation bays where it is stabilised by adding pure carbon dioxide gas to the water. This lowers the p. H to between 8, 0 and 8. 4. 15 15

5. Filtration The water passes into the filter houses where it flows through rapid gravity sand filter beds of finely graded silica sand pebbles. The remaining suspended particles are removed at this stage. 16 16

6. Chlorination The water leaving the purification plant is disinfected with chlorine to kill micro-organisms, bacteria and viruses that may be present in the water. 17 17

Water Supply This clean water is pumped through underground pipes into reservoirs that supply homes, schools, businesses and factories with clean water. 18 18

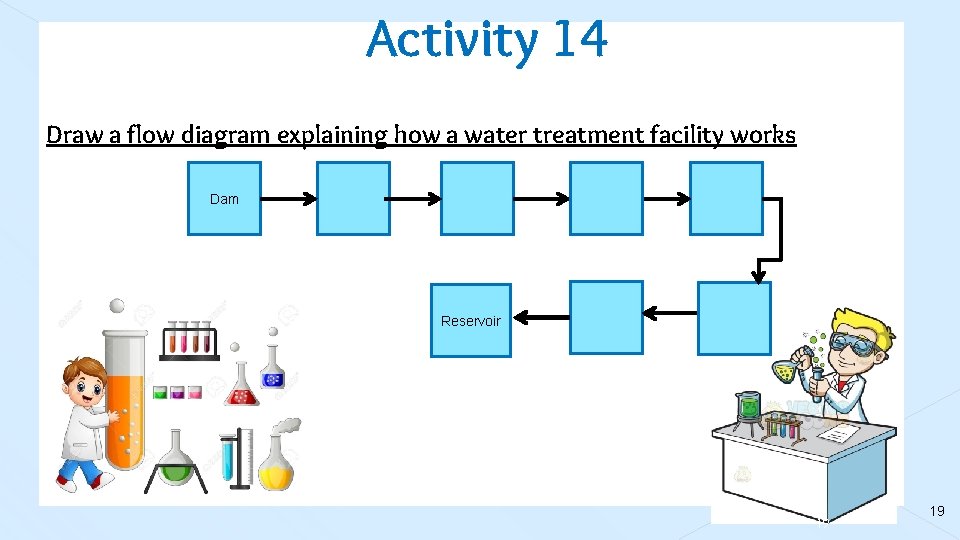
Activity 14 Draw a flow diagram explaining how a water treatment facility works Dam Reservoir 19 19

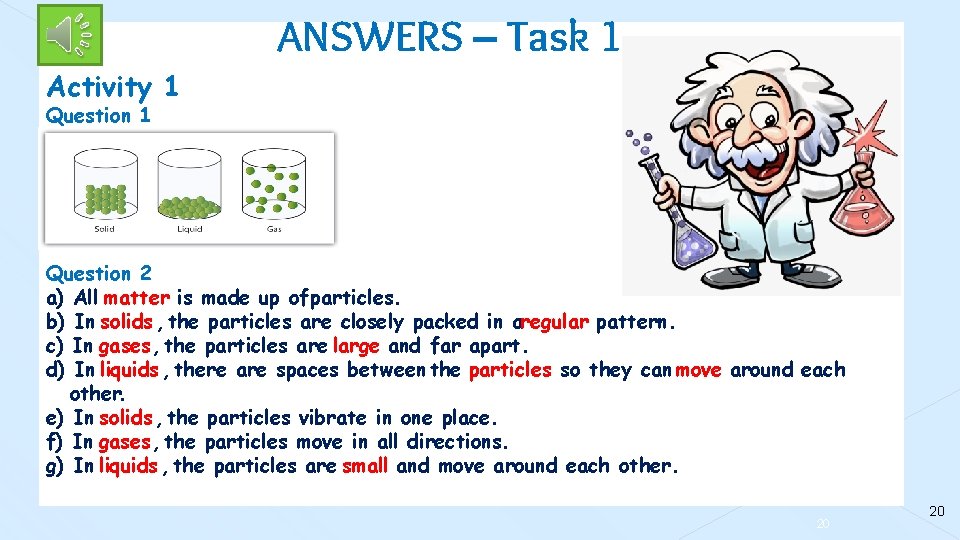
ANSWERS – Task 1 Activity 1 Question 2 a) All matter is made up of particles. b) In solids , the particles are closely packed in aregular pattern. c) In gases, the particles are large and far apart. d) In liquids , there are spaces between the particles so they can move around each other. e) In solids , the particles vibrate in one place. f) In gases, the particles move in all directions. g) In liquids , the particles are small and move around each other. 20 20

Activity 2 State what you think the best method to separate each of the following mixtures are: A – beans and lentils - hand sorting B - flour and rice - sieving C - sand water - filtering D - oil and water - settling & decanting 21

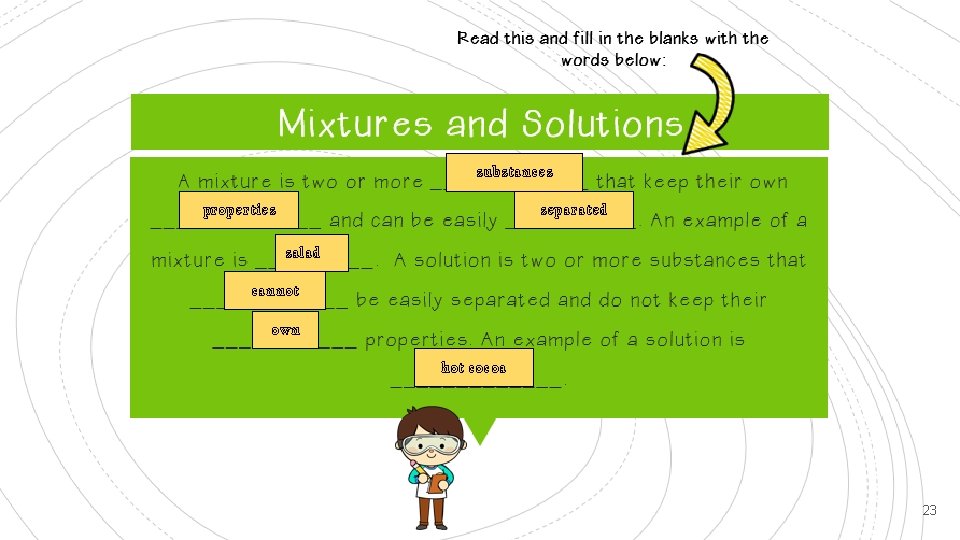
ANSWERS – Task 2 Lesson 3 A mixture is a combination of two or more substances that keep their own properties and can easily be separated A solution is a combination of two or more substances that do not keep their own properties and cannot be easily be separated. They are uniform in appearance. Beans and samp coffee 22

substances properties separated salad cannot own hot cocoa 23

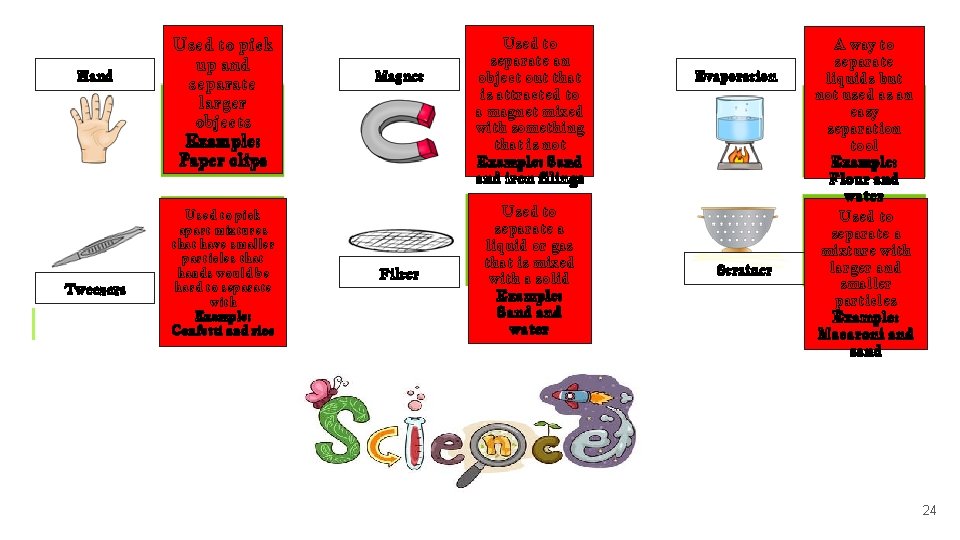
Hand Tweezers Used to pick up and separate larger objects Example: Paper clips Used to pick apart mixtures that have smaller particles that hands would be hard to separate with Example: Confetti and rice Magnet Filter Used to separate an object out that is attracted to a magnet mixed with something that is not Example: Sand iron filings Used to separate a liquid or gas that is mixed with a solid Example: Sand water Evaporation Strainer A way to separate liquids but not used as an easy separation tool Example: Flour and water Used to separate a mixture with larger and smaller particles Example: Macaroni and sand 24

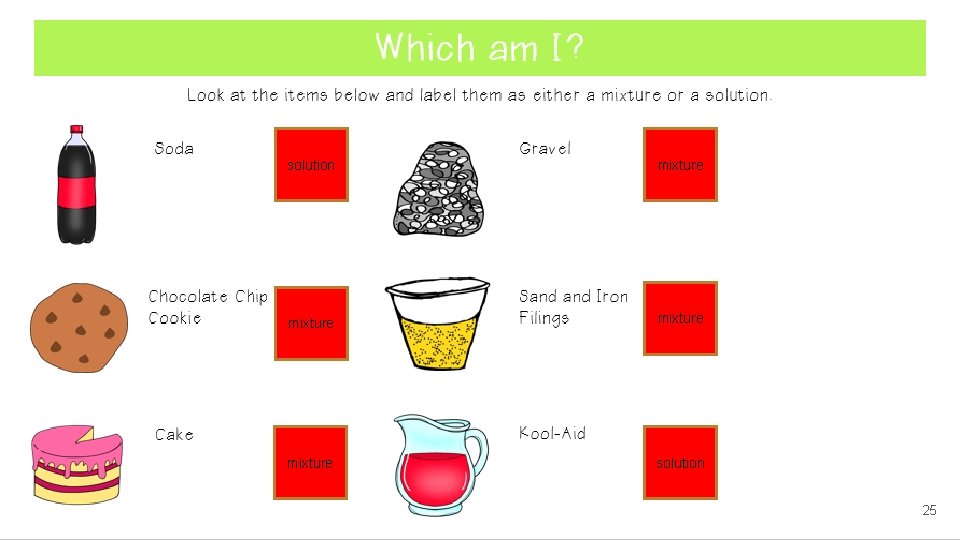
solution mixture solution 25

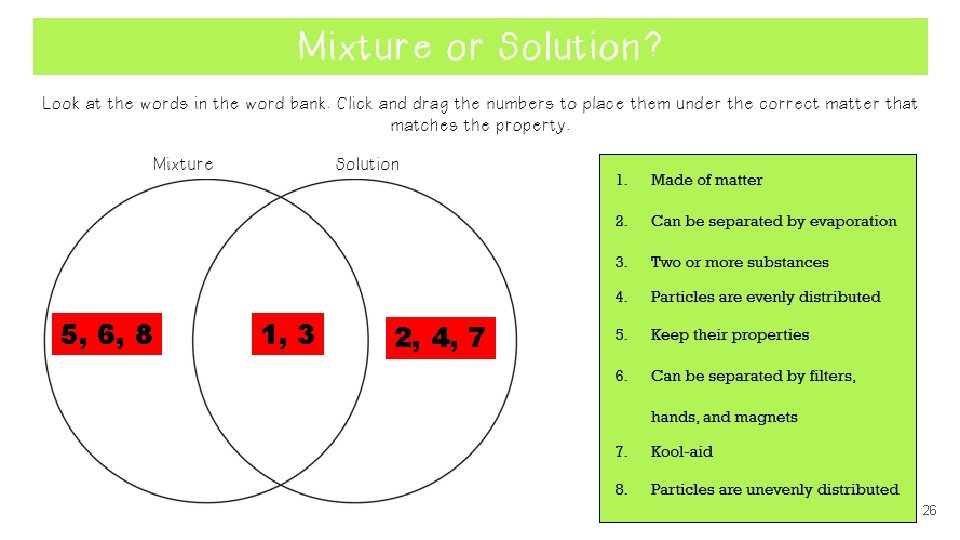
5, 6, 8 1, 3 2, 4, 7 26

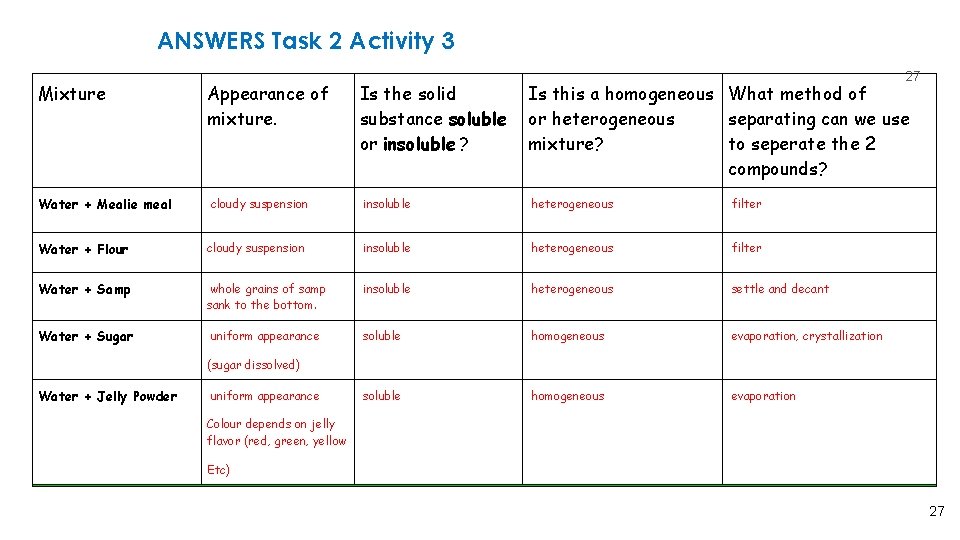
ANSWERS Task 2 Activity 3 Mixture Water + Mealie meal Appearance of mixture. cloudy suspension Is the solid substance soluble or insoluble ? 27 Is this a homogeneous What method of or heterogeneous separating can we use mixture? to seperate the 2 compounds? insoluble heterogeneous filter Water + Flour cloudy suspension insoluble heterogeneous filter Water + Samp whole grains of samp sank to the bottom. insoluble heterogeneous settle and decant Water + Sugar uniform appearance soluble homogeneous evaporation, crystallization soluble homogeneous evaporation (sugar dissolved) Water + Jelly Powder uniform appearance Colour depends on jelly flavor (red, green, yellow Etc) 27

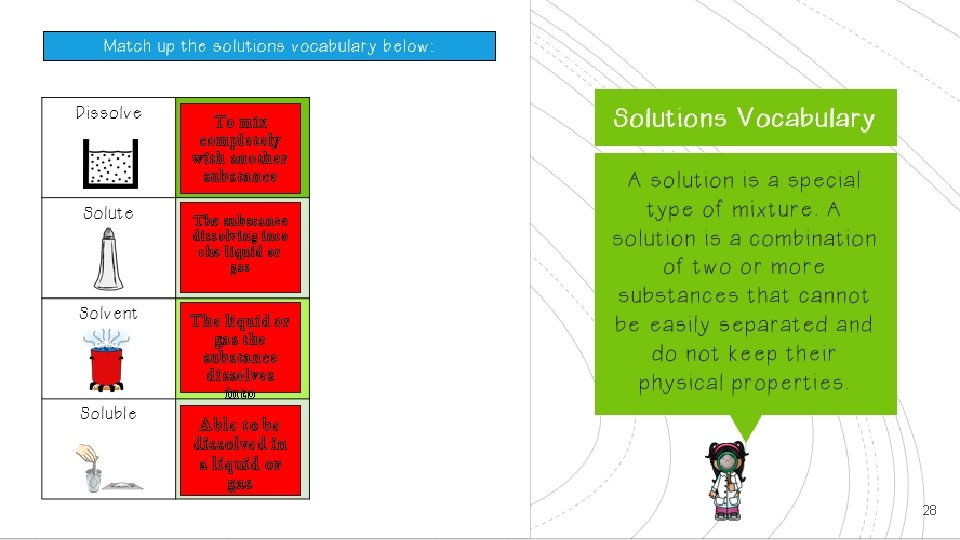
To mix completely with another substance The substance dissolving into the liquid or gas The liquid or gas the substance dissolves into Able to be dissolved in a liquid or gas 28

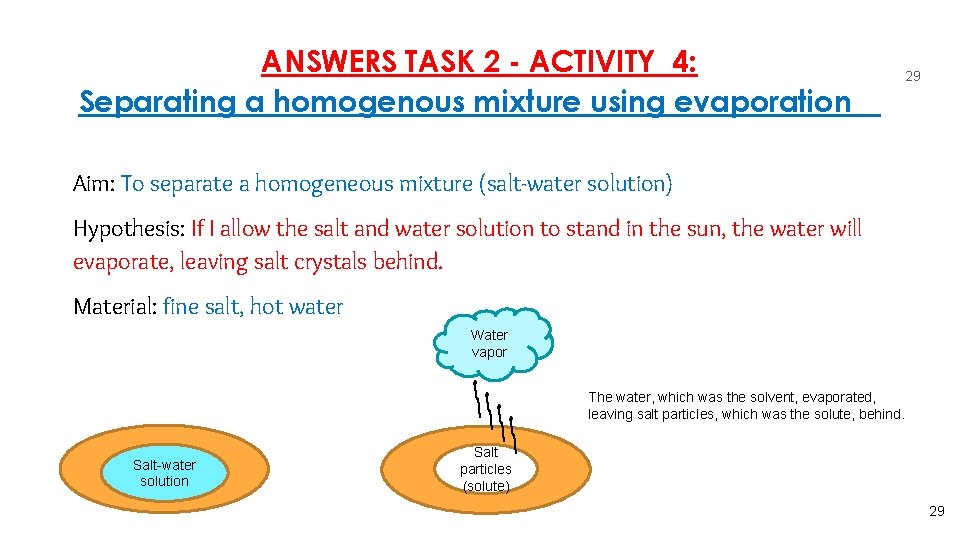
ANSWERS TASK 2 - ACTIVITY 4: Separating a homogenous mixture using evaporation 29 Aim: To separate a homogeneous mixture (salt-water solution) Hypothesis: If I allow the salt and water solution to stand in the sun, the water will evaporate, leaving salt crystals behind. Material: fine salt, hot water Water vapor The water, which was the solvent, evaporated, leaving salt particles, which was the solute, behind. Salt-water solution Salt particles (solute) 29

Answers Task 3 Activity 5 1. How long did it take for crystals to start forming on the string? (subject to learners experiment) 2. What are the crystals made of? Sugar 3. Why do you think we boiled the water when dissolving the sugar in the solution? Boiling the water increases the temperature of the solvent which allows more solute to dissolve. 30

Answers Task 3 Activity 6 1. What is a heterogeneous mixture? It is the combination of two or more substances where, both substances retain their properties and are easily separated. They do not have a uniform appearance. 2. Which separation method worked the best? Filtering 3. What could we add to the funnel to improve filtration? (ie. to remove more sand particles from water) a layer of gravel and fine sand, additional cotton wool 31

Answers Task 3 Lesson 7 1. What is this process called? It is called filtration. 2. Why do the sand grains stay behind on the filter paper, but the water passes through it? The sand particles are too large to pass through the filter paper. 3. What was the mixture of sugar and water called? (Hint: It was a special kind of mixture called a. . ? ) solution / homogeneous mixture 4. What would happen if the mixture of sugar and water is poured through a filter? The sugar has dissolved in the water, the sugar particles are dispersed among the water particles, the sugar particles will pass through the filter paper along with the water. 32

5. Would it be possible to separate the water and the sugar? Yes, by allowing the water to evaporate, the sugar will crystallize. 6. What happens to the sugar when it dissolves in the water? The solute(sugar) particles are dispersed between the solvent (water)particles. 7. Why is it not possible to separate a solution through a filter? The solute particles are dispersed among the solvent particles. Both particles will be small enough to pass through the filter paper. 8. Describe how you can get the solid sugar back from the sugar solution. If you allow the solvent(water) to evaporate, the solute(sugar) will remain behind (crystallize). 33