Picture 1 Picture 2 Picture 3 Picture 4

















- Slides: 26

Picture 1

Picture 2

Picture 3

Picture 4

Picture 5

STATES OF MATTER • Matter can be classified into groups based on the shape and volume of their moving particles – SOLID – LIQUID – GAS – PLASMA

• SOLID - state of matter when materials have definite shape and definite volume – Atoms are packed tightly together – Atoms are arranged neatly, orderly – Atoms vibrate around the same location

Examples of Solids are • • • Ice Frog Cheese Bricks Wood Popcicle

• Liquid- state of matter when materials have definite volume and indefinite shape – Liquid takes the same shape as its container – Particles FLOW past one another easily – Atoms are close, but not tight – Atoms are randomly arranged

Examples of Liquids are • • • Orange Juice Water Soft drinks Milk Rubbing Alcohol Vinegar

• Gas – state of matter when materials have indefinite shape AND indefinite volume – Take shape and volume of container – Atoms spread to fit container – Atoms move rapidly, constantly – Atoms randomly arranged with large spaces between them

Examples of Gases are • • • Steam Oxygen Carbon dioxide Smog Tear Gas Helium

• Plasma – state of matter made up of small electrically charged particles – Found where there is high temperature and pressure – Rare to find on Earth – Ex, lightning – Used in fluorescent light bulbs and neon lights

MATTER • Kinetic Energy (KE) - energy of motion – All matter is made of millions of tiny particles – these particles are constantly moving, and have KE

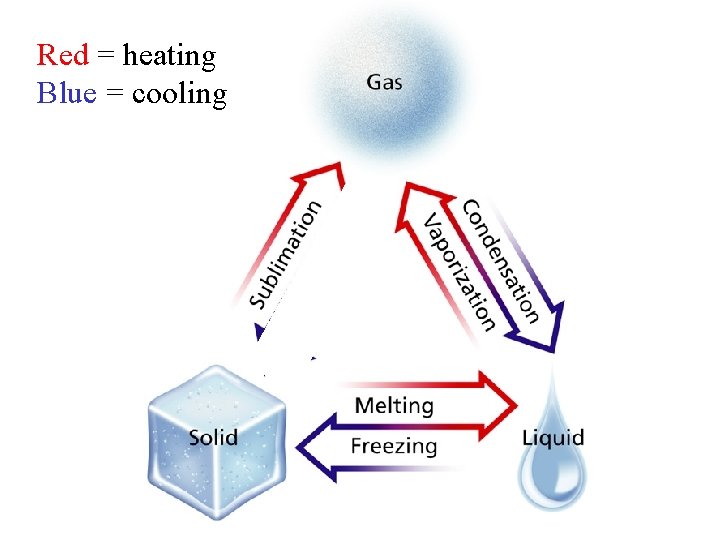
• Phase Change - reversible physical change from one state to another – heat energy is absorbed or released

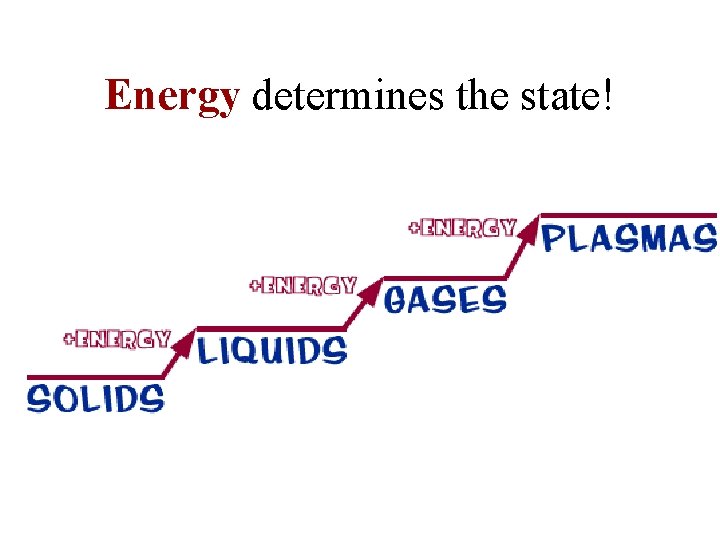
Energy determines the state!

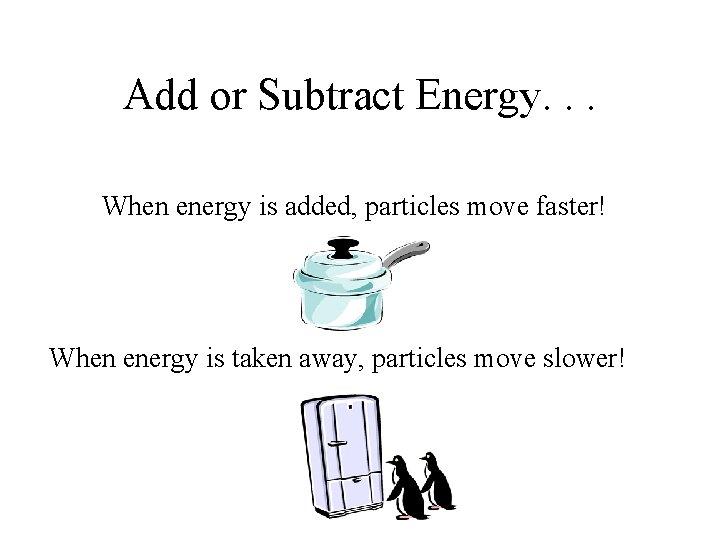
Add or Subtract Energy. . . When energy is added, particles move faster! When energy is taken away, particles move slower!

Red = heating Blue = cooling Bla hbl ah bla hbl ahb hla

• Melting – changing from solid to liquid – When a solid gains heat – Temperature and energy INCREASE

• Freezing – changing from liquid to solid – When a liquid loses heat – Baked cookies are actually FROZEN because they are solid. – Freezing does not always mean COLD. – Freezing point and melting point are the same

• Evaporation – changing from a liquid to a gas – Temperature is below boiling point – Water VAPOR or STEAM = gas – More area = faster evaporation

• Boiling – when vapor pressure is the same as atmospheric pressure and bubbling occurs – Vapor pressure - pressure created by moving gas particles bumping into each other or the container – Atmospheric pressure – pressure outside the container

• Condensation – change from gas to liquid – Water vapor particles hit a cool surface, lose heat, and change into water • Sublimation- change from solid to gas – NO LIQUID IN BETWEEN – Dry ice

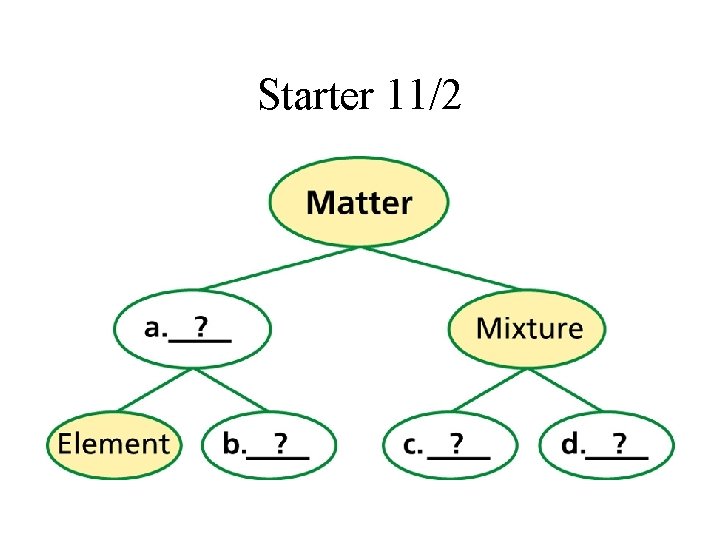
Starter 11/2

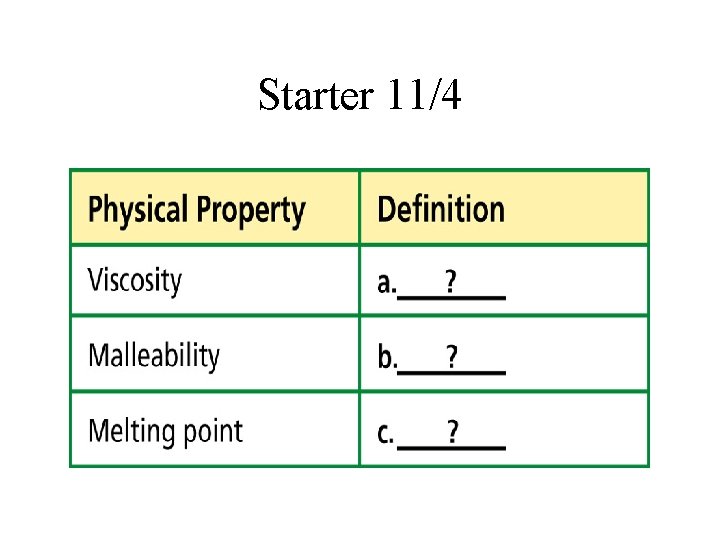
Starter 11/4

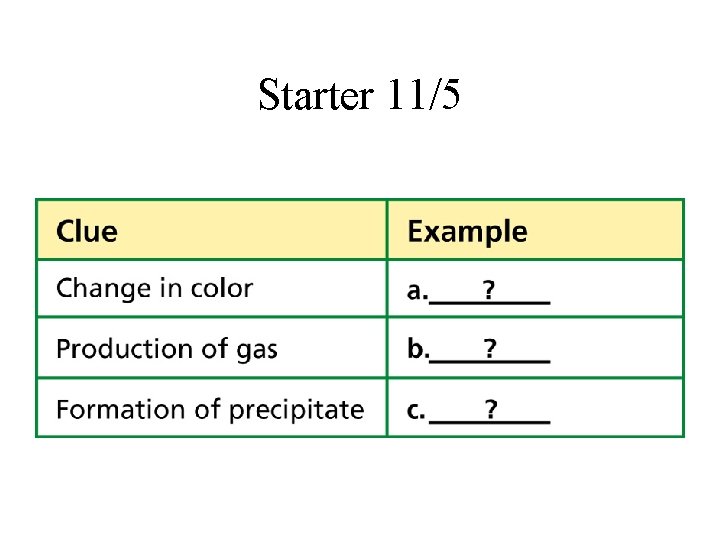
Starter 11/5