Pick up notes Get out Ionic Nomenclature practice










- Slides: 16

§ Pick up notes. § Get out Ionic Nomenclature practice.

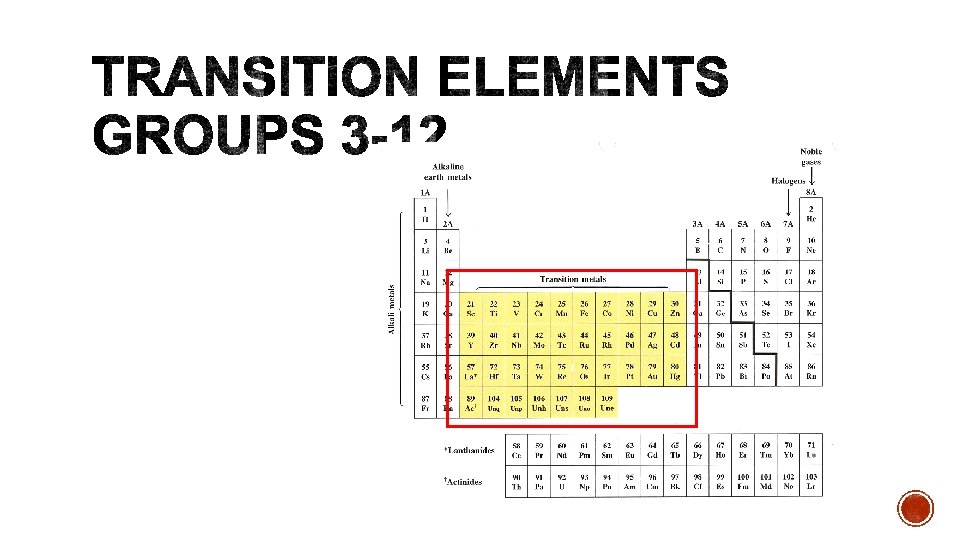
§ Some cations can have more than one charge. § Most are transition elements. Lead and tin are exceptions. § Example: Cu+1 and Cu+2. § It is important to distinguish which ion is in the compound.


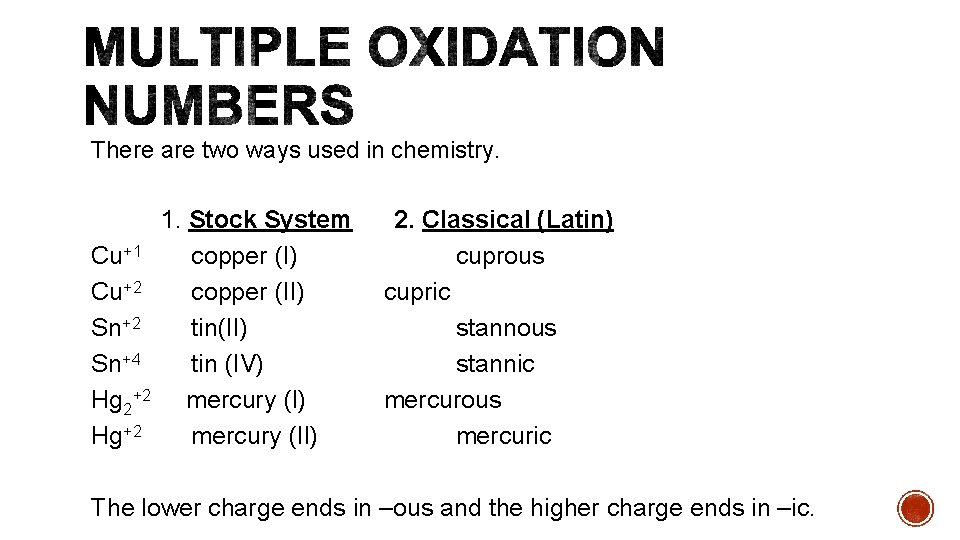
There are two ways used in chemistry. Cu+1 Cu+2 Sn+4 Hg 2+2 Hg+2 1. Stock System copper (I) copper (II) tin (IV) mercury (II) 2. Classical (Latin) cuprous cupric stannous stannic mercurous mercuric The lower charge ends in –ous and the higher charge ends in –ic.

You are responsible in class for both systems, but only the Stock system will be on the test.

cupric oxide/copper (II) oxide Cu. O ferrous sulfide/iron (II) sulfide Fe. S cobaltic bromide/cobalt (III) bromide Co. Br 3 mercurous chloride/mercury (I) chloride Hg 2 Cl 2

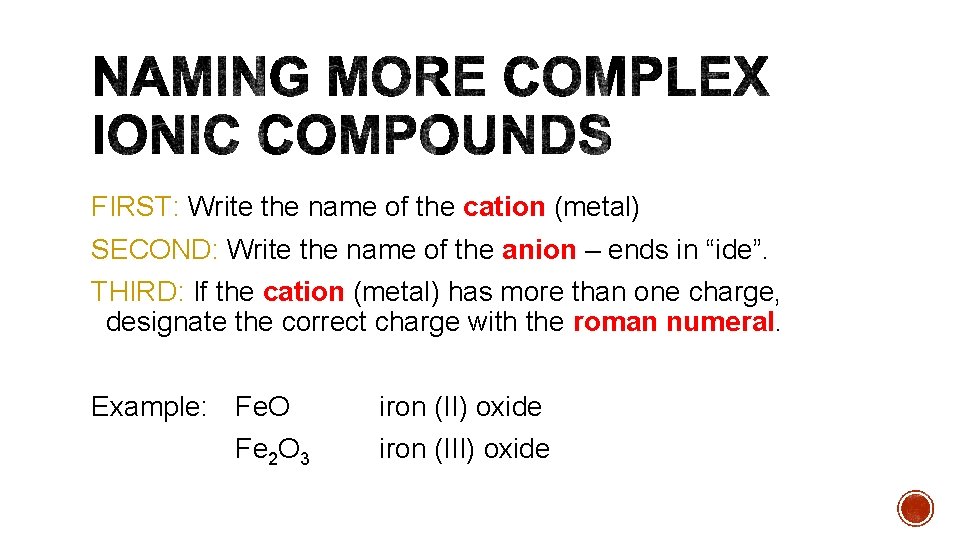
FIRST: Write the name of the cation (metal) SECOND: Write the name of the anion – ends in “ide”. THIRD: If the cation (metal) has more than one charge, designate the correct charge with the roman numeral. Example: Fe. O Fe 2 O 3 iron (II) oxide iron (III) oxide

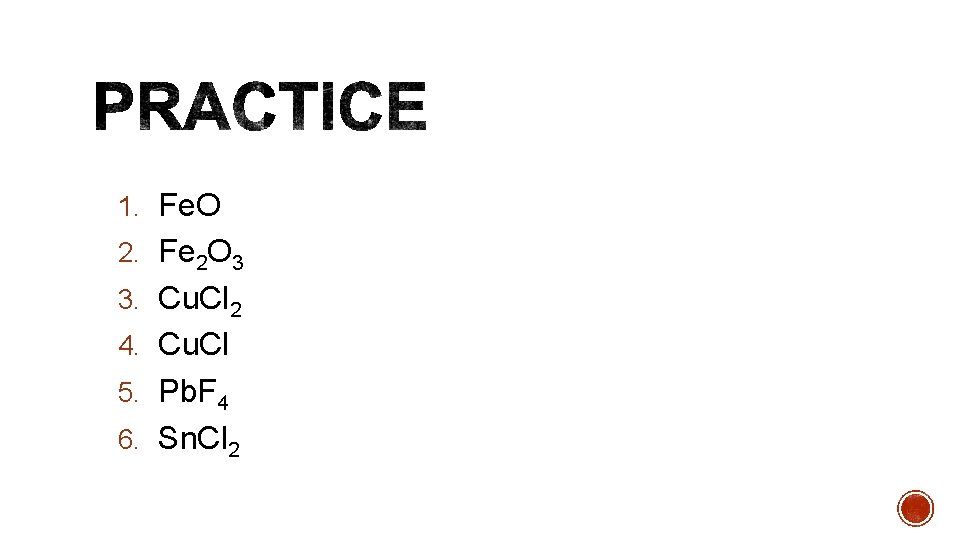
1. Fe. O 2. Fe 2 O 3 3. Cu. Cl 2 4. Cu. Cl 5. Pb. F 4 6. Sn. Cl 2

• FIRST: Write the cation with the correct charge (given in the name). • SECOND: Write the anion with the correct charge. Copper(II) chloride Cu+2 and Cl-1 Cu. Cl 2

1. copper(I) fluoride 2. copper(II) oxide 3. chromium(III) bromide 4. manganese(II) iodide 5. mercury(II) chloride 6. cobalt(III) sulfide

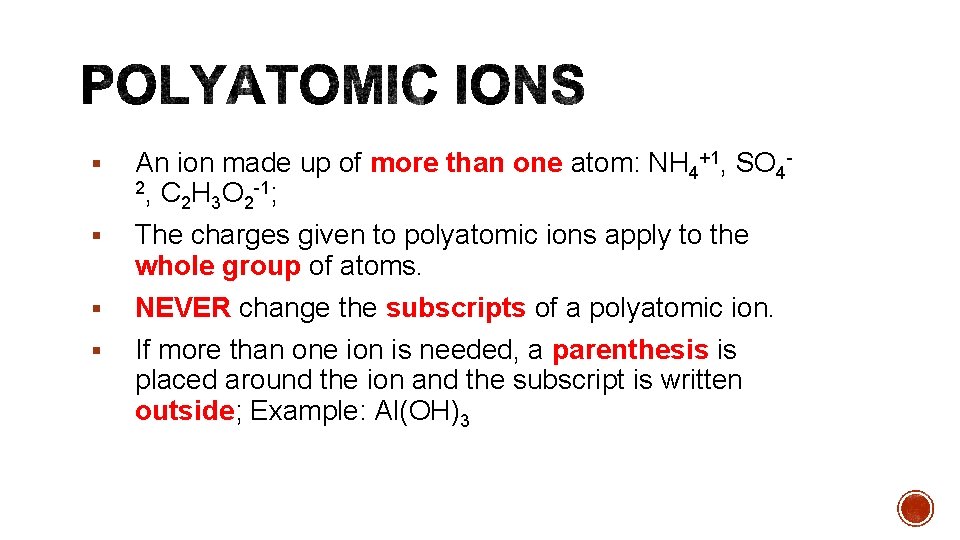
§ An ion made up of more than one atom: NH 4+1, SO 42, C H O -1; 2 3 2 § The charges given to polyatomic ions apply to the whole group of atoms. § NEVER change the subscripts of a polyatomic ion. § If more than one ion is needed, a parenthesis is placed around the ion and the subscript is written outside; Example: Al(OH)3

FIRST: Write the name of the cation (metal). If it gets a roman numeral, be sure to write it. SECOND: Write the name of the anion. If it is a polyatomic ion, use the name given on your ion sheet.

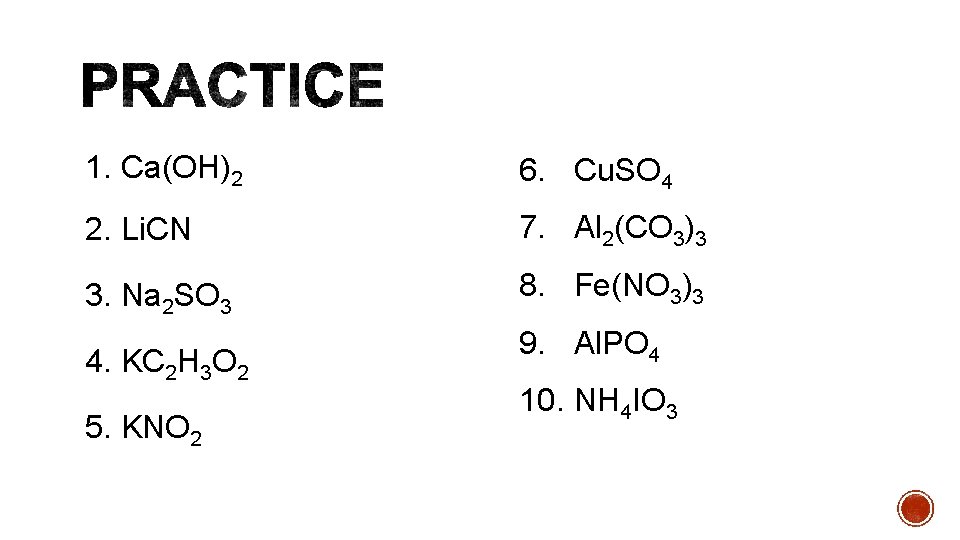
1. Ca(OH)2 6. Cu. SO 4 2. Li. CN 7. Al 2(CO 3)3 3. Na 2 SO 3 8. Fe(NO 3)3 4. KC 2 H 3 O 2 5. KNO 2 9. Al. PO 4 10. NH 4 IO 3

§ Write the symbol for the cation, then the symbol for the anion. § Balance the charges - use the crisscross method - by placing subscripts and “( )” for polyatomic ions if needed. § Monatomic ions don’t need “( )”. § Net charges must be zero. Use ion sheet to get charges.

1. Potassium iodate 6. Potassium sulfate 2. Iron (II) hydroxide 7. Copper (II) sulfite 3. Iron (III) oxalate 8. Barium cyanide 4. Beryllium silicate 9. copper (II) sulfite 5. Sodium acetate 10. tin (IV) chlorate

Do the PRACTICE PROBLEMS, backside of Ionic Nomenclature Practice, for tomorrow.