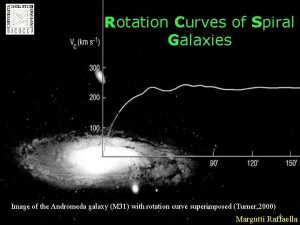
Physics the science of matter and energy and









- Slides: 31

Physics: the science of matter and energy and interactions between the two, grouped in traditional field like acoustics, optics, mechanics, thermodynamics, and electromagnetism, etc. Mechanics: motion and its causes, interactions between objects Thermodynamics: heat and temperature Vibrations and wave phenomena: specific types of repetitive motions Optics: light Electromagnetism: electricity, magnetism and light Relativity: particles moving at any speed and includes very high speeds Quantum mechanics: behavior of submicroscopic particles

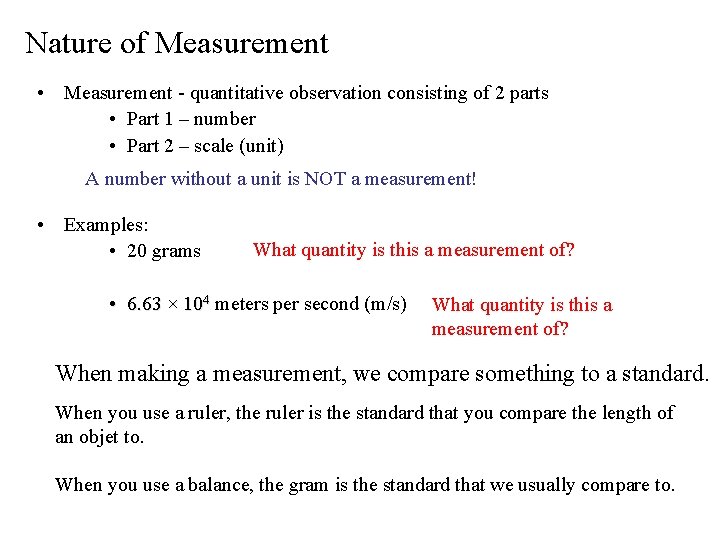
Nature of Measurement • Measurement - quantitative observation consisting of 2 parts • Part 1 – number • Part 2 – scale (unit) A number without a unit is NOT a measurement! • Examples: What quantity is this a measurement of? • 20 grams • 6. 63 × 104 meters per second (m/s) What quantity is this a measurement of? When making a measurement, we compare something to a standard. When you use a ruler, the ruler is the standard that you compare the length of an objet to. When you use a balance, the gram is the standard that we usually compare to.

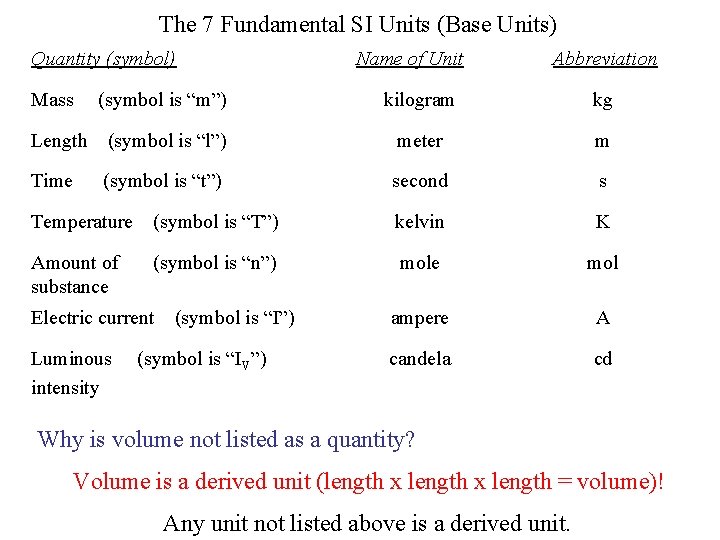
The 7 Fundamental SI Units (Base Units) Quantity (symbol) Name of Unit Abbreviation Mass (symbol is “m”) kilogram kg Length (symbol is “l”) meter m Time (symbol is “t”) second s Temperature (symbol is “T”) kelvin K Amount of (symbol is “n”) substance mol Electric current (symbol is “I”) ampere A Luminous (symbol is “IV”) intensity candela cd Why is volume not listed as a quantity? Volume is a derived unit (length x length = volume)! Any unit not listed above is a derived unit.

Derived Units Derived units are units that are defined by a combination of two or more base units. Volume = (length x width x height) If distances are in meters, what would the units for volume be? (m)x(m) = m 3 (cubic meters) A definition: 1 cm 3 = 1 m. L (one cubic centimeter is one milliliter) Density = mass ÷ volume = m/V If mass is in grams and volume is in m. L, what would the units for density be? (g)÷(m. L) = g/m. L (gram per milliliter)

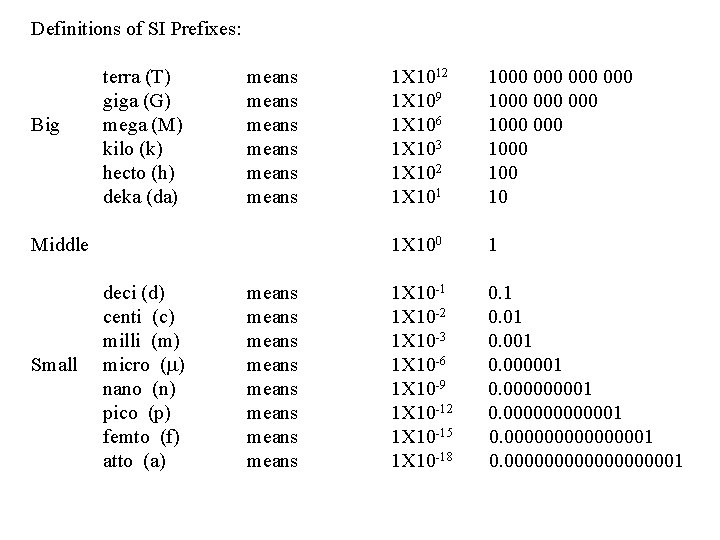
Definitions of SI Prefixes: terra (T) means giga (G) means Big mega (M) means kilo (k) means hecto (h) means deka (da) means 1 X 1012 1000 000 1 X 109 1000 000 1 X 106 1000 1 X 103 1000 1 X 102 100 1 X 101 10 Middle 1 X 100 Small deci (d) centi (c) milli (m) micro (m) nano (n) pico (p) femto (f) atto (a) means means 1 1 X 10 -1 0. 1 1 X 10 -2 0. 01 1 X 10 -3 0. 001 1 X 10 -6 0. 000001 1 X 10 -9 0. 00001 1 X 10 -12 0. 0000001 1 X 10 -15 0. 00000001 1 X 10 -18 0. 0000000001

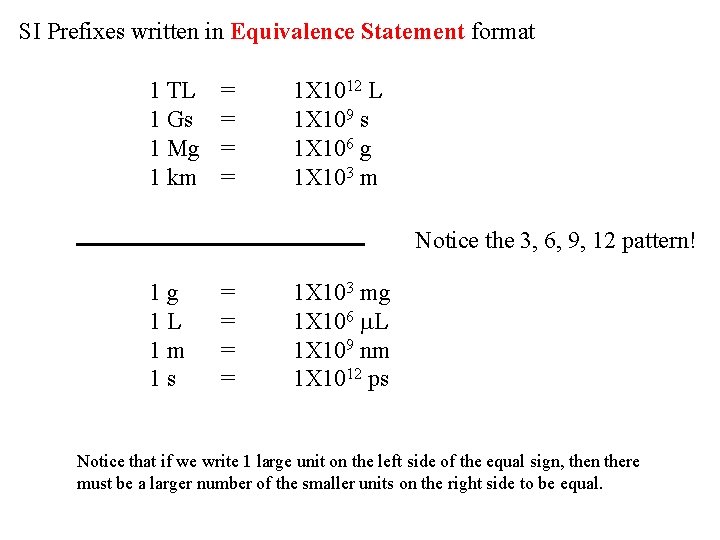
SI Prefixes written in Equivalence Statement format 1 TL 1 Gs 1 Mg 1 km = = 1 X 1012 L 1 X 109 s 1 X 106 g 1 X 103 m Notice the 3, 6, 9, 12 pattern! 1 g 1 L 1 m 1 s = = 1 X 103 mg 1 X 106 m. L 1 X 109 nm 1 X 1012 ps Notice that if we write 1 large unit on the left side of the equal sign, then there must be a larger number of the smaller units on the right side to be equal.

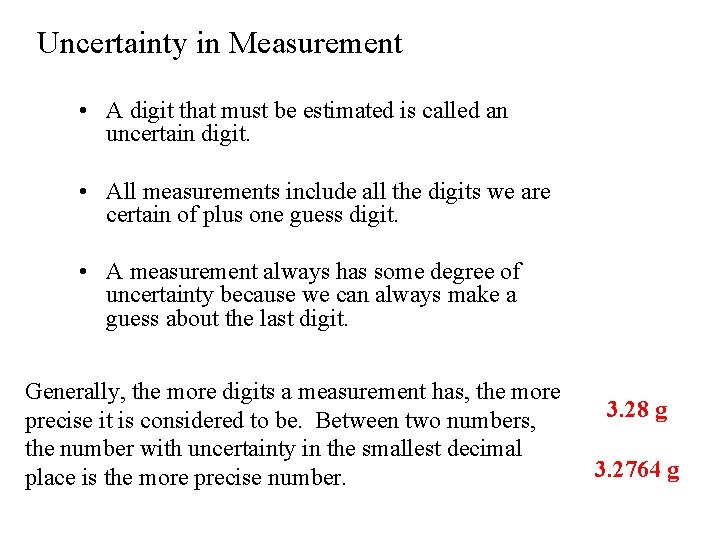
Uncertainty in Measurement • A digit that must be estimated is called an uncertain digit. • All measurements include all the digits we are certain of plus one guess digit. • A measurement always has some degree of uncertainty because we can always make a guess about the last digit. Generally, the more digits a measurement has, the more precise it is considered to be. Between two numbers, the number with uncertainty in the smallest decimal place is the more precise number. 3. 28 g 3. 2764 g

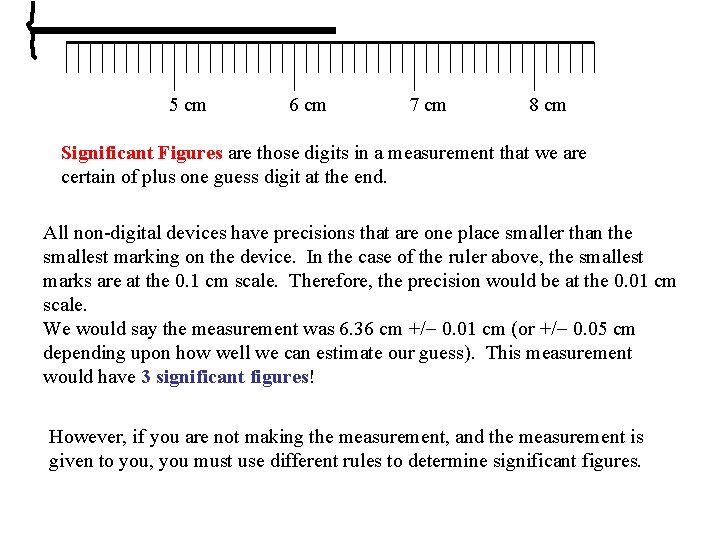
5 cm 6 cm 7 cm 8 cm Significant Figures are those digits in a measurement that we are certain of plus one guess digit at the end. All non-digital devices have precisions that are one place smaller than the smallest marking on the device. In the case of the ruler above, the smallest marks are at the 0. 1 cm scale. Therefore, the precision would be at the 0. 01 cm scale. We would say the measurement was 6. 36 cm +/- 0. 01 cm (or +/- 0. 05 cm depending upon how well we can estimate our guess). This measurement would have 3 significant figures! However, if you are not making the measurement, and the measurement is given to you, you must use different rules to determine significant figures.

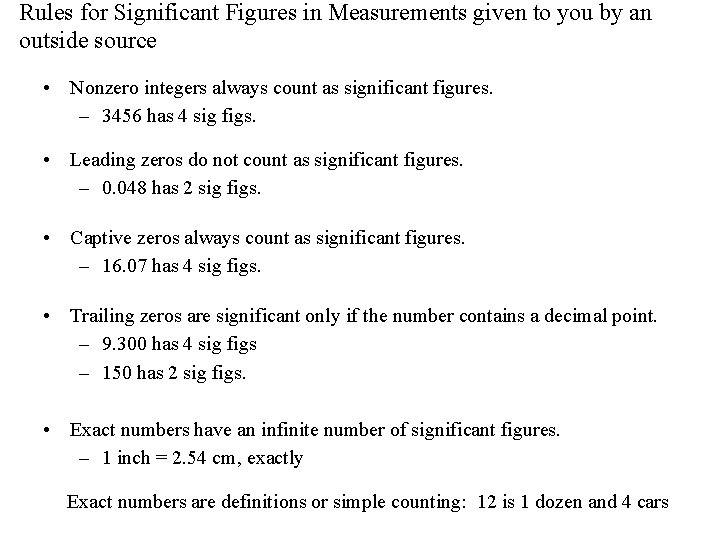
Rules for Significant Figures in Measurements given to you by an outside source • Nonzero integers always count as significant figures. – 3456 has 4 sig figs. • Leading zeros do not count as significant figures. – 0. 048 has 2 sig figs. • Captive zeros always count as significant figures. – 16. 07 has 4 sig figs. • Trailing zeros are significant only if the number contains a decimal point. – 9. 300 has 4 sig figs – 150 has 2 sig figs. • Exact numbers have an infinite number of significant figures. – 1 inch = 2. 54 cm, exactly Exact numbers are definitions or simple counting: 12 is 1 dozen and 4 cars

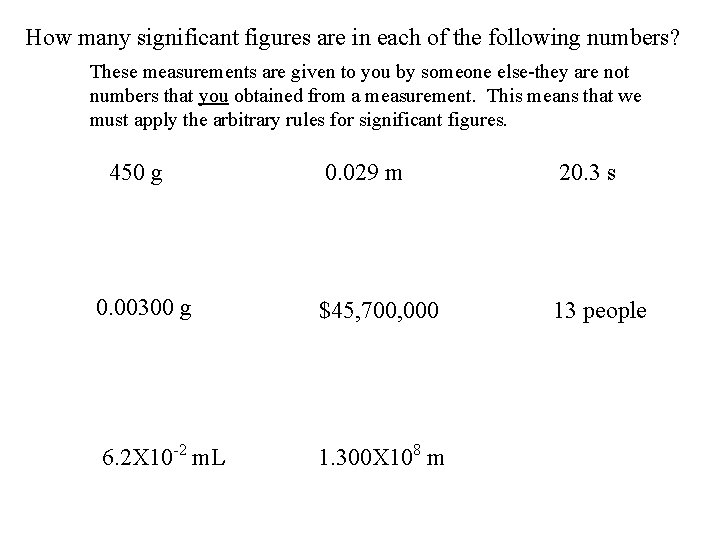
How many significant figures are in each of the following numbers? These measurements are given to you by someone else-they are not numbers that you obtained from a measurement. This means that we must apply the arbitrary rules for significant figures. 450 g 0. 029 m 20. 3 s 0. 00300 g $45, 700, 000 13 people 6. 2 X 10 -2 m. L 1. 300 X 108 m

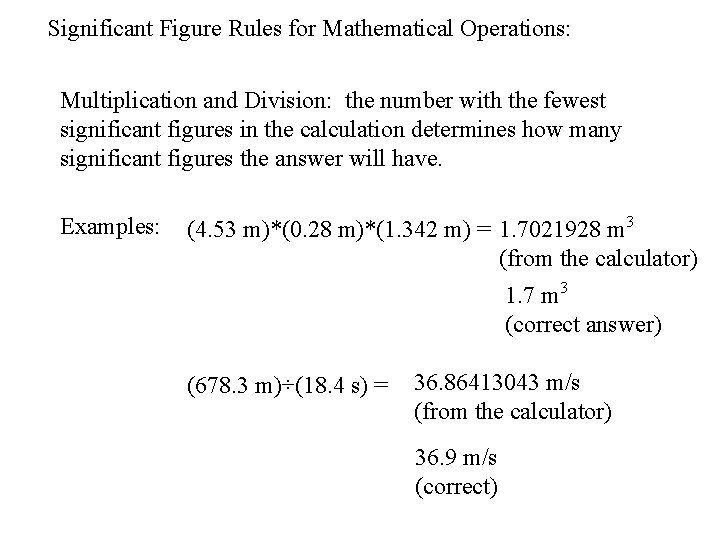
Significant Figure Rules for Mathematical Operations: Multiplication and Division: the number with the fewest significant figures in the calculation determines how many significant figures the answer will have. Examples: (4. 53 m)*(0. 28 m)*(1. 342 m) = 1. 7021928 m 3 (from the calculator) 1. 7 m 3 (correct answer) (678. 3 m)÷(18. 4 s) = 36. 86413043 m/s (from the calculator) 36. 9 m/s (correct)

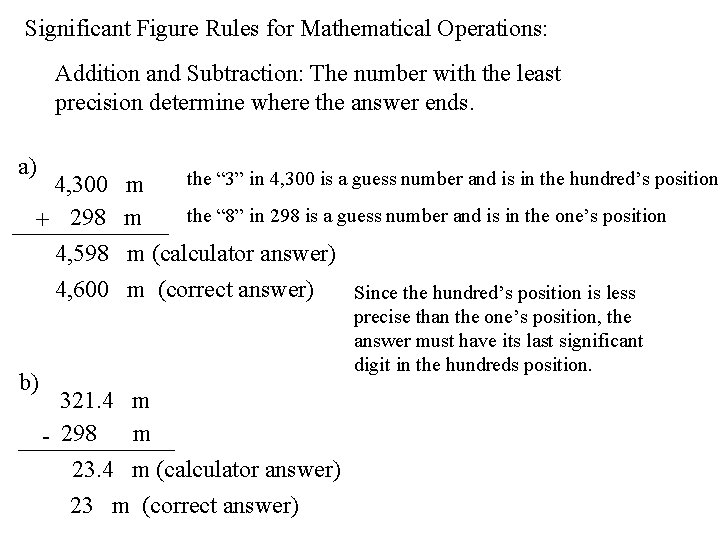
Significant Figure Rules for Mathematical Operations: Addition and Subtraction: The number with the least precision determine where the answer ends. a) the “ 3” in 4, 300 is a guess number and is in the hundred’s position 4, 300 m the “ 8” in 298 is a guess number and is in the one’s position + 298 m 4, 598 m (calculator answer) 4, 600 m (correct answer) Since the hundred’s position is less b) precise than the one’s position, the answer must have its last significant digit in the hundreds position. 321. 4 m - 298 m 23. 4 m (calculator answer) 23 m (correct answer)

Scientific Method: review (discussion)

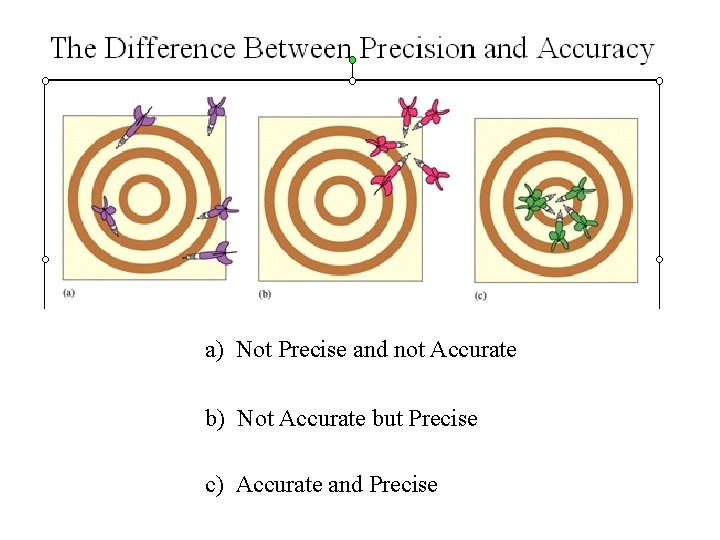
a) Not Precise and not Accurate b) Not Accurate but Precise c) Accurate and Precise

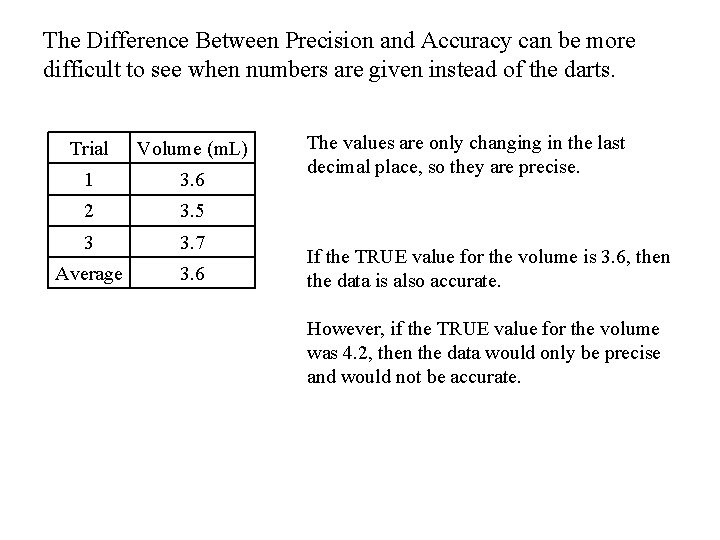
The Difference Between Precision and Accuracy can be more difficult to see when numbers are given instead of the darts. Trial Volume (m. L) 1 3. 6 2 3. 5 3 3. 7 Average 3. 6 The values are only changing in the last decimal place, so they are precise. If the TRUE value for the volume is 3. 6, then the data is also accurate. However, if the TRUE value for the volume was 4. 2, then the data would only be precise and would not be accurate.

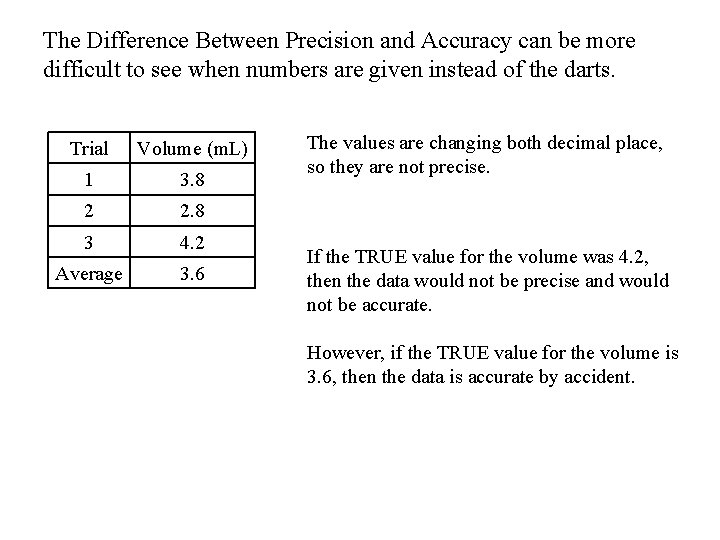
The Difference Between Precision and Accuracy can be more difficult to see when numbers are given instead of the darts. Trial Volume (m. L) 1 3. 8 2 2. 8 3 4. 2 Average 3. 6 The values are changing both decimal place, so they are not precise. If the TRUE value for the volume was 4. 2, then the data would not be precise and would not be accurate. However, if the TRUE value for the volume is 3. 6, then the data is accurate by accident.

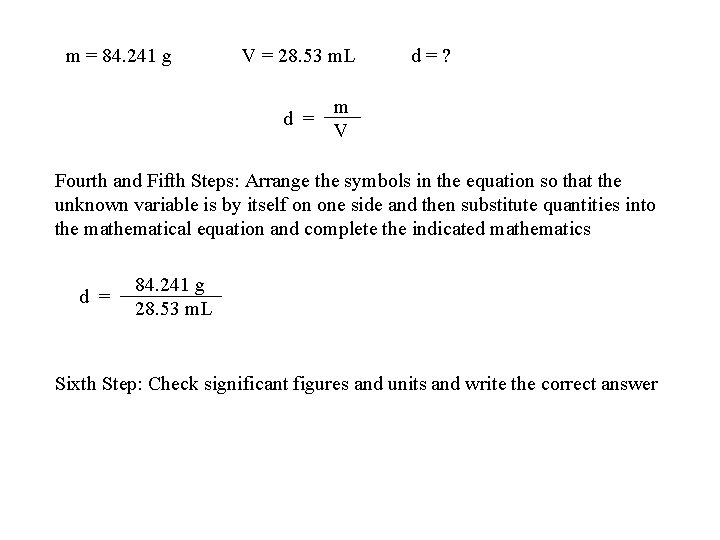
Problem Solving Strategy Illustration A solid object is found to have a mass of 84. 241 g and a volume of 28. 53 m. L. What is the density of the object? First Step: Highlight key concepts or quantities in the word problem Second Step: Assign an appropriate symbol for all key quantities mass = m = 84. 241 g volume = V = 28. 53 m. L density = d = ? Third Step: Use the list of symbols to identify any useful equations m d = V

m = 84. 241 g V = 28. 53 m. L d = ? m d = V Fourth and Fifth Steps: Arrange the symbols in the equation so that the unknown variable is by itself on one side and then substitute quantities into the mathematical equation and complete the indicated mathematics 84. 241 g d = 28. 53 m. L Sixth Step: Check significant figures and units and write the correct answer

You have 14. 3 m. L of an object that has a density of 7. 932 g/m. L. What is the mass of the object? You have 435. 3 g of a liquid that has a density of 0. 8325 g/m. L. What is the volume of the liquid?

Problem Solving Strategy Illustration 2 See Example Problem 1 on page 4 What is the resistance of a light bulb that has a current of 0. 75 amp and a voltage of 120 v? From the book the key equation is: V = IR Where V is voltage in volts (v) I is current in amperes (amp) R is resistance in ohms (ohm) Use the approach outlined in the 1 st Problem Solving Strategy Illustration and the information on page 4 to complete the problem above.

Mathematics with Scientific Notation: Let your calculator handle the exponents! Let’s do an example: 3. 4 X 106 + 2. 8 X 105 On your calculator (Texas Instruments), type the following in order: 3. 4 2 nd EE 6 + 2. 8 2 nd EE 5 On your calculator (Casio), type the following in order: 3. 4 exp 6 + 2. 8 exp 5 = When you let the calculator handle the math, you job is to take care of the significant figures-calculators do not understand significant figures! =

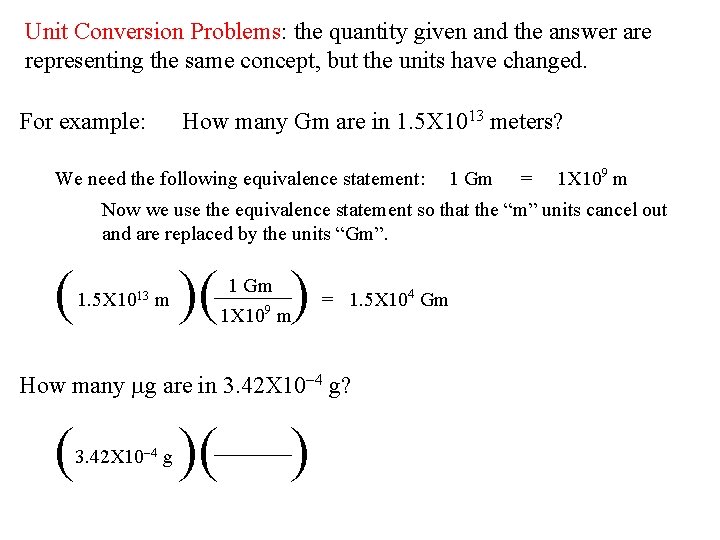
Unit Conversion Problems: the quantity given and the answer are representing the same concept, but the units have changed. For example: How many Gm are in 1. 5 X 1013 meters? We need the following equivalence statement: 1 Gm = 1 X 109 m Now we use the equivalence statement so that the “m” units cancel out and are replaced by the units “Gm”. ( 1. 5 X 1013 m )( ) 1 Gm 1 X 109 m = 1. 5 X 104 Gm How many mg are in 3. 42 X 10 -4 g? ( 3. 42 X 10 -4 g )( )

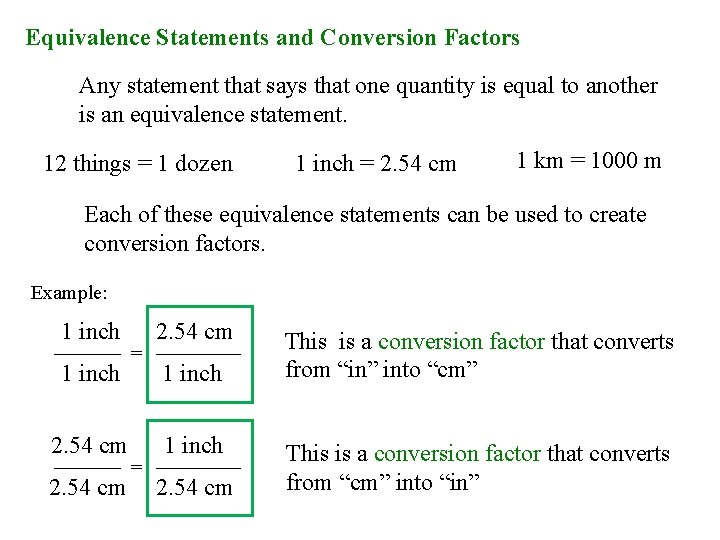
Equivalence Statements and Conversion Factors Any statement that says that one quantity is equal to another is an equivalence statement. 12 things = 1 dozen 1 inch = 2. 54 cm 1 km = 1000 m Each of these equivalence statements can be used to create conversion factors. Example: 1 inch 2. 54 cm = 1 inch 2. 54 cm 1 inch = 2. 54 cm This is a conversion factor that converts from “in” into “cm” This is a conversion factor that converts from “cm” into “in”

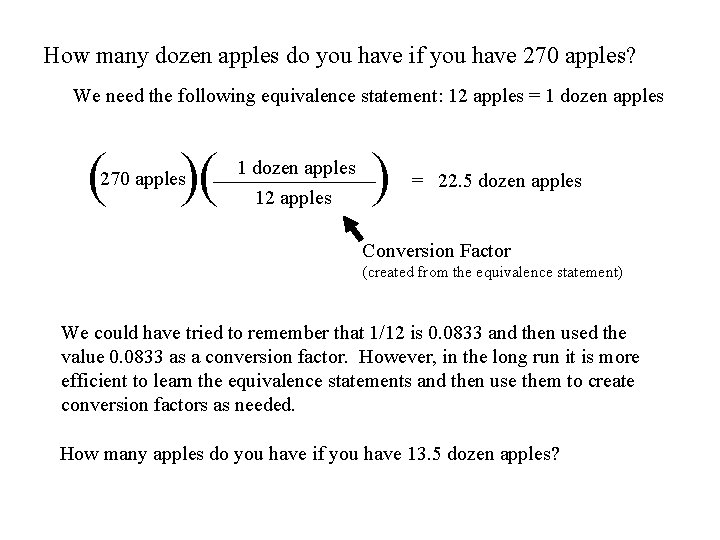
How many dozen apples do you have if you have 270 apples? We need the following equivalence statement: 12 apples = 1 dozen apples ( )( 270 apples 1 dozen apples 12 apples ) = 22. 5 dozen apples Conversion Factor (created from the equivalence statement) We could have tried to remember that 1/12 is 0. 0833 and then used the value 0. 0833 as a conversion factor. However, in the long run it is more efficient to learn the equivalence statements and then use them to create conversion factors as needed. How many apples do you have if you have 13. 5 dozen apples?

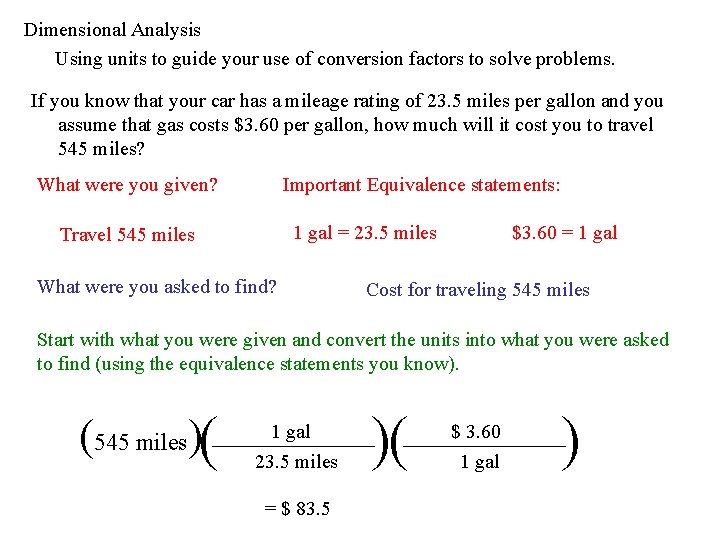
Dimensional Analysis Using units to guide your use of conversion factors to solve problems. If you know that your car has a mileage rating of 23. 5 miles per gallon and you assume that gas costs $3. 60 per gallon, how much will it cost you to travel 545 miles? What were you given? Important Equivalence statements: 1 gal = 23. 5 miles $3. 60 = 1 gal Travel 545 miles What were you asked to find? Cost for traveling 545 miles Start with what you were given and convert the units into what you were asked to find (using the equivalence statements you know). (545 miles)( 1 gal 23. 5 miles = $ 83. 5 )( $ 3. 60 1 gal )

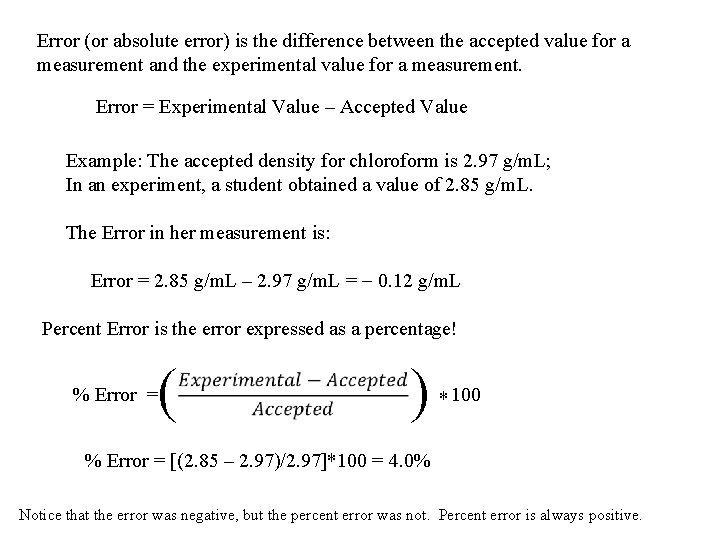
Error (or absolute error) is the difference between the accepted value for a measurement and the experimental value for a measurement. Error = Experimental Value – Accepted Value Example: The accepted density for chloroform is 2. 97 g/m. L; In an experiment, a student obtained a value of 2. 85 g/m. L. The Error in her measurement is: Error = 2. 85 g/m. L – 2. 97 g/m. L = - 0. 12 g/m. L Percent Error is the error expressed as a percentage! % Error = ( ) * 100 % Error = [(2. 85 – 2. 97)/2. 97]*100 = 4. 0% Notice that the error was negative, but the percent error was not. Percent error is always positive.

Independent Variable: the variable that a scientist chooses to change during an experiment is called the independent variable Dependent Variable: the variable that responds to changes in made to the independent variable is called the dependent variable When making a graph, we plot the independent variable on the x-axis (horizontal) and the dependent variable on the y-axis. If this type of graph forms a line, then the dependent and independent variable have a linear relationship which can be described by the general equation for a line: y = mx + b where m is the slope and b is the y intercept Slope = ( y 2 – y 1 x 2 – x 1 )

Non-linear Relationships Non-linear relationships fall into two categories: Quadratic Relationships and Inverse Relationships If the best fit for the data is an equation like this: y = ax 2 + bx + c then the data has a quadratic relationship and the shape of the graph is called a parabola. If the best fit for the data is an equation like this: y = a/x then the data has an inverse relationship and the shape of the graph is called a hyperbola Note: if the data does not form any type of discernible pattern the variables are not related to each other (in other words, changing one does not do anything particular to the other.

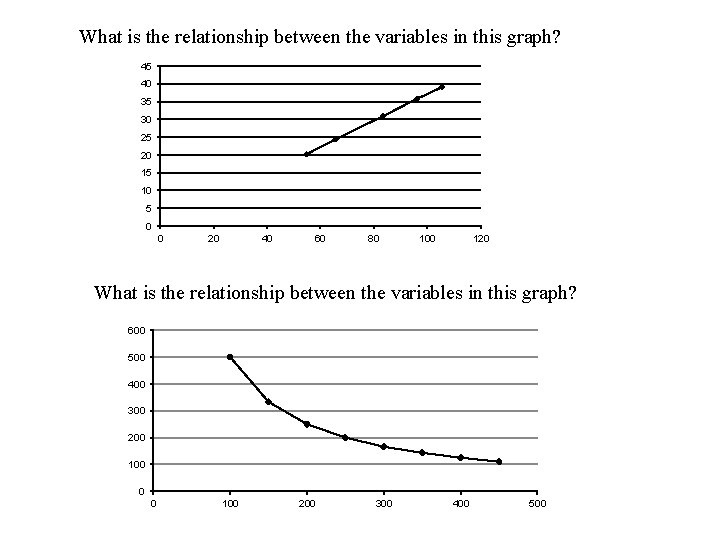
What is the relationship between the variables in this graph? 45 40 35 30 25 20 15 10 5 0 0 20 40 60 80 100 120 What is the relationship between the variables in this graph? 600 500 400 300 200 100 0 0 100 200 300 400 500

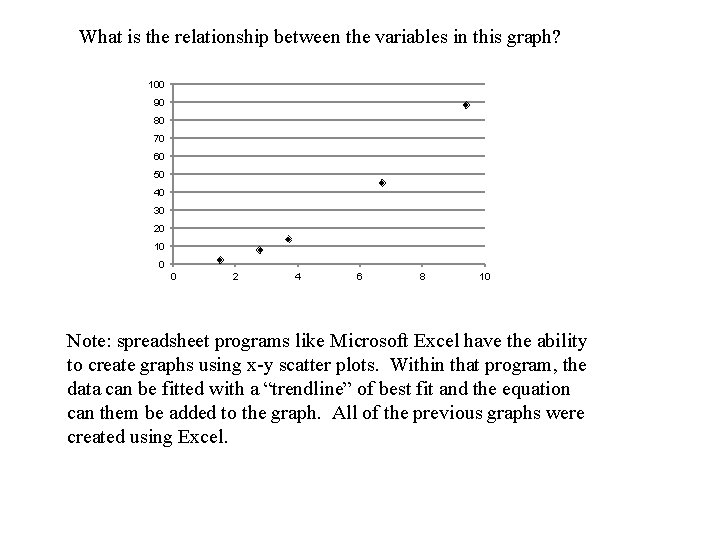
What is the relationship between the variables in this graph? 100 90 80 70 60 50 40 30 20 10 0 0 2 4 6 8 10 Note: spreadsheet programs like Microsoft Excel have the ability to create graphs using x-y scatter plots. Within that program, the data can be fitted with a “trendline” of best fit and the equation can them be added to the graph. All of the previous graphs were created using Excel.

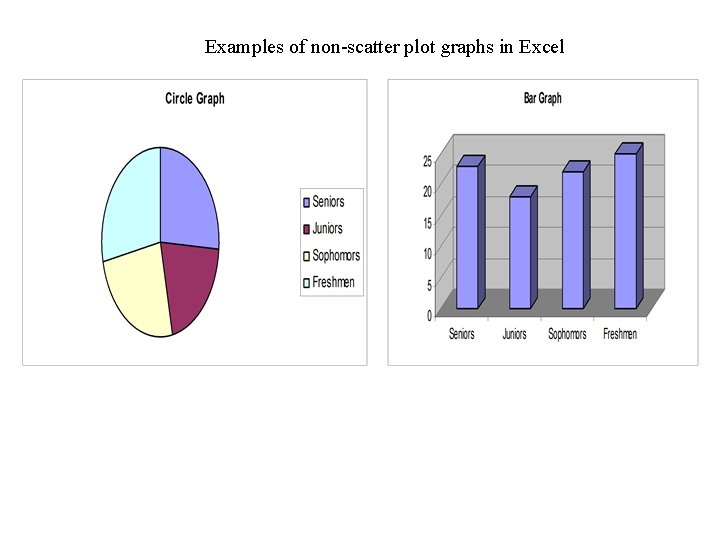
Examples of non-scatter plot graphs in Excel
 Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Science matter
Science matter What are your favorite subjects
What are your favorite subjects Gray matter in the brain
Gray matter in the brain Gray matter and white matter
Gray matter and white matter Gray matter and white matter
Gray matter and white matter What is grey matter
What is grey matter Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Dark matter physics
Dark matter physics Grade 7 natural science separating mixtures
Grade 7 natural science separating mixtures Natural science grade 7 lesson plans term 2
Natural science grade 7 lesson plans term 2 The science of deformation and flow of matter
The science of deformation and flow of matter Natural science and technology grade 6 term 2
Natural science and technology grade 6 term 2 Lesson 3 energy and matter in ecosystems answer key
Lesson 3 energy and matter in ecosystems answer key Which reverses the normal flow of thermal energy
Which reverses the normal flow of thermal energy Matter and thermal energy section 1
Matter and thermal energy section 1 Matter energy and measurement
Matter energy and measurement Dark matter and dark energy ppt
Dark matter and dark energy ppt Unit 2 matter and energy
Unit 2 matter and energy Trophic levels examples
Trophic levels examples Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Phase change concept map solid liquid gas
Phase change concept map solid liquid gas Phases of matter foldable
Phases of matter foldable Mechanical wave examples
Mechanical wave examples Kesler science electromagnetic spectrum answer key
Kesler science electromagnetic spectrum answer key Tertiary consumer defintion
Tertiary consumer defintion Why does it happen
Why does it happen