Physics REVISION Work Energy transfer diagrams Different types



- Slides: 13

Physics REVISION – Work, Energy transfer diagrams Different types of energy can be transferred from one type to another. Energy transfer diagrams show energy types in and out of a process. Conservation of Energy: Energy cannot be destroyed or created, but it can change from one form to another. Everyday Examples: Law of Conservation of Energy. The law of conservation of energy can be seen in these everyday examples of energy transference: 1. Water can produce electricity. Water falls from the sky, converting potential energy to kinetic energy. This energy is then used to rotate the turbine of a generator to produce electricity. In this process, the potential energy of water in a dam can be turned into kinetic energy which can then become electric energy. 2. When playing pool, the cue ball is shot at a stationary 8 ball. The cue ball has energy. When the cue ball hits the 8 ball, the energy transfers from the cue ball to the 8 ball, sending the 8 ball into motion. The cue ball loses energy because the energy it had has been transferred to the 8 ball, so the cue ball slows down. 3. Kelly ran across the room and bumped into her brother, pushing him to the floor. The kinetic energy she possessed because of her movement was transferred to her brother, causing him to move. 4. A cat sitting on the highest branch of a tree has what is known as potential energy. If he falls off the branch and falls to the ground, his potential energy is now being converted into kinetic energy.

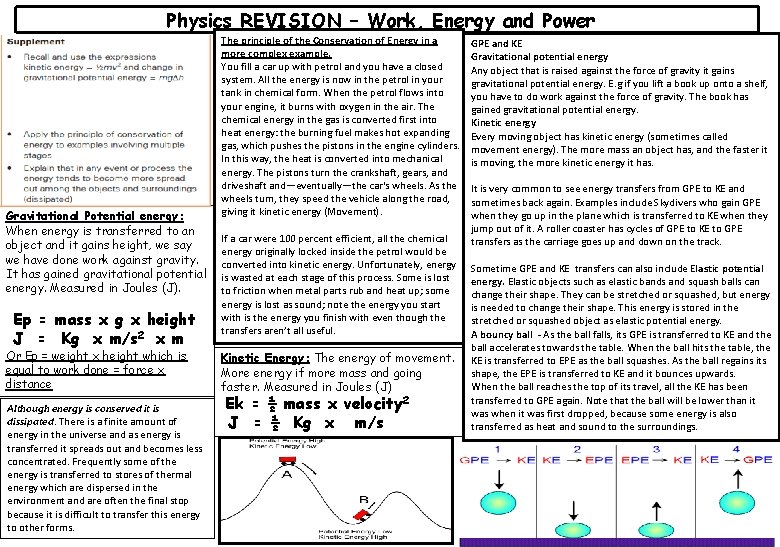
Physics REVISION – Work, Energy and Power Gravitational Potential energy: When energy is transferred to an object and it gains height, we say we have done work against gravity. It has gained gravitational potential energy. Measured in Joules (J). Ep = mass x g x height J = Kg x m/s 2 x m Or Ep = weight x height which is equal to work done = force x distance Although energy is conserved it is dissipated. There is a finite amount of energy in the universe and as energy is transferred it spreads out and becomes less concentrated. Frequently some of the energy is transferred to stores of thermal energy which are dispersed in the environment and are often the final stop because it is difficult to transfer this energy to other forms. The principle of the Conservation of Energy in a more complex example. You fill a car up with petrol and you have a closed system. All the energy is now in the petrol in your tank in chemical form. When the petrol flows into your engine, it burns with oxygen in the air. The chemical energy in the gas is converted first into heat energy: the burning fuel makes hot expanding gas, which pushes the pistons in the engine cylinders. In this way, the heat is converted into mechanical energy. The pistons turn the crankshaft, gears, and driveshaft and—eventually—the car's wheels. As the wheels turn, they speed the vehicle along the road, giving it kinetic energy (Movement). If a car were 100 percent efficient, all the chemical energy originally locked inside the petrol would be converted into kinetic energy. Unfortunately, energy is wasted at each stage of this process. Some is lost to friction when metal parts rub and heat up; some energy is lost as sound; note the energy you start with is the energy you finish with even though the transfers aren’t all useful. Kinetic Energy: The energy of movement. More energy if more mass and going faster. Measured in Joules (J) Ek = ½ mass x velocity 2 J = ½ Kg x m/s GPE and KE Gravitational potential energy Any object that is raised against the force of gravity it gains gravitational potential energy. E. g if you lift a book up onto a shelf, you have to do work against the force of gravity. The book has gained gravitational potential energy. Kinetic energy Every moving object has kinetic energy (sometimes called movement energy). The more mass an object has, and the faster it is moving, the more kinetic energy it has. It is very common to see energy transfers from GPE to KE and sometimes back again. Examples include Skydivers who gain GPE when they go up in the plane which is transferred to KE when they jump out of it. A roller coaster has cycles of GPE to KE to GPE transfers as the carriage goes up and down on the track. Sometime GPE and KE transfers can also include Elastic potential energy. Elastic objects such as elastic bands and squash balls can change their shape. They can be stretched or squashed, but energy is needed to change their shape. This energy is stored in the stretched or squashed object as elastic potential energy. A bouncy ball - As the ball falls, its GPE is transferred to KE and the ball accelerates towards the table. When the ball hits the table, the KE is transferred to EPE as the ball squashes. As the ball regains its shape, the EPE is transferred to KE and it bounces upwards. When the ball reaches the top of its travel, all the KE has been transferred to GPE again. Note that the ball will be lower than it was when it was first dropped, because some energy is also transferred as heat and sound to the surroundings.

Physics REVISION – Energy Efficient Example Work done: When energy is transferred we say we have done work. Work is done when a force is used to move an object. Work is measured in Joules (J) Work = force x distance moved in the direction of the force Energy Efficiency: Efficiency is a way of describing how good a device is at transferring energy from one form to another in an intended way. If a light bulb is rated 100 W, this means that it normally uses 100 J of electrical energy every second. But it might only produce 5 J of light energy every second. The other 95 J are wasted as heat. This means that only 5% of the energy is transferred from electrical energy into light energy. This light bulb therefore has an efficiency of 0. 05 or 5%. If the same light bulb were used as a heater, its efficiency would be 95% or 0. 95, because the intended output energy form would be heat, not light. Two equivalent equations which can be used to define efficiency. Since efficiency is a ratio of two quantities each with the same unit, efficiency itself is dimensionless, that is, efficiency has no unit. During an energy transfer energy efficiency is dependent on how much useful energy is produced in comparison to the total energy input. Very few energy transfers are 100% efficient. To calculate efficiency: efficiency = useful energy out/total energy in (for a decimal efficiency) efficiency = useful energy out/total energy in)× 100% (for a percentage efficiency) Calculating efficiency example 1 – Weightlifter A weightlifter raises a bar and masses totally 120 kg a distance of 2. 2 m above his head. Work done or energy is calculated by Force x distance Step 1 – turn the mass in weight (force) so 120 Kg x 10 = 1200 N Work done/energy = 1200 N x 2. 2 m = 2, 460 J The weightlifter used 3, 500 J of energy to raise the weight – how efficient was he? Efficiency = energy out/ energy in = 2, 460/3, 500 = 0. 754 Wasted Energy: In most energy transfers some energy is transferred to a wasted form of energy. Heat and Sound are the 2 most common forms of wasted energy. Example 2 – Heat Engine A heat engine gives out 400 J of heat energy as the useful work. Calculate the energy given to it as input if its efficiency is 40%? Given: Energy output = 400 J, Energy Input = ? Efficiency = 40 % Efficiency = Energy Output/ Energy Input × 100 % ∴ Energy input = Energy Output / Efficiency = 4000/40 = 1000 J

Physics REVISION – Work, Energy and Power Renewal vs Non Renewal continued Renewal sources of electricity, once used can be easily produced again eg Wood can be regrown or Solar which is available every day. Non-renewal on the other hand cannot easily be replaced. Eg Coal takes millions of years to form. Nuclear Fusion The Sun – Our main energy resource The chemical energy from the sun contributes to all the fossil fuels. Plants use sunlight to photosynthesis and grow. The eventually die and decay and over millions of years create fossil fuels. The sun also heavily contributes to the weather. Winds are created by the rising and cooling of air particles. This also contributes towards the creation waves meaning the sun is vital for solar, wave and wind power. . Renewal vs Non Renewal – Pro’s and Con’s Non renewable sources produce large amounts of energy, in particular Nuclear power. There are large quantities of Uranium available however the Nuclear waste produce remains radioactive for years and Nuclear power is expensive to keep safe. When fossil fuels are burnt they release Carbon Dioxide and Sulphur Dioxide which contribute towards Global Warming and Acid rain respectively. All fossil fuel supplies are running out. Renewal sources are expensive to set up and don’t produce large amounts of electricity. They are cheap once up and running and have low fuel costs. They will last for an infinite amount of time and, with the exception of wood, they do not produce pollution in the form of CO 2 and SO 2. Nuclear fusion is the process of forcing two nuclei close enough together so they form a single larger nucleus. Nuclear Fusion happens in the Sun when 2 Hydrogen atoms combine to produce a larger Helium nucleus. It is a clean form of producing large amount of energy. The Hydrogen fuel can be taken from water. However to recreate Nuclear Fusion it requires extremely high temperatures and pressure which is hard to recreate in a stable environment.

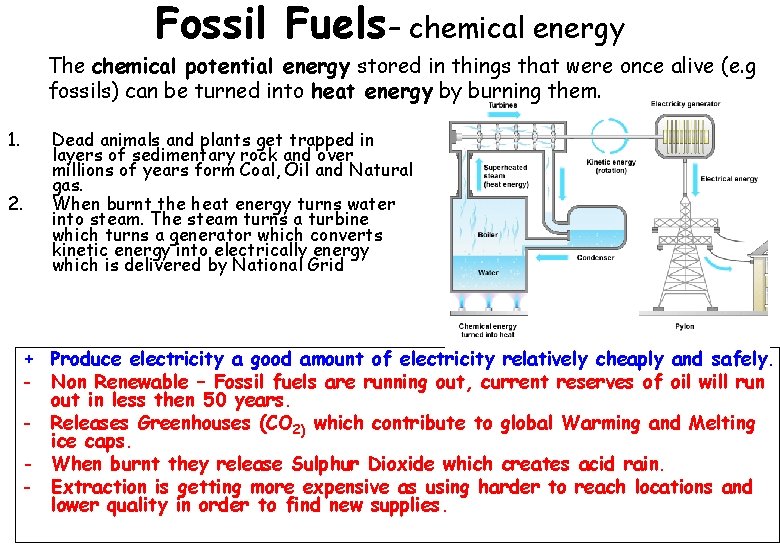
Fossil Fuels– chemical energy The chemical potential energy stored in things that were once alive (e. g fossils) can be turned into heat energy by burning them. 1. 2. Dead animals and plants get trapped in layers of sedimentary rock and over millions of years form Coal, Oil and Natural gas. When burnt the heat energy turns water into steam. The steam turns a turbine which turns a generator which converts kinetic energy into electrically energy which is delivered by National Grid + Produce electricity a good amount of electricity relatively cheaply and safely. - Non Renewable – Fossil fuels are running out, current reserves of oil will run out in less then 50 years. - Releases Greenhouses (CO 2) which contribute to global Warming and Melting ice caps. - When burnt they release Sulphur Dioxide which creates acid rain. - Extraction is getting more expensive as using harder to reach locations and lower quality in order to find new supplies.

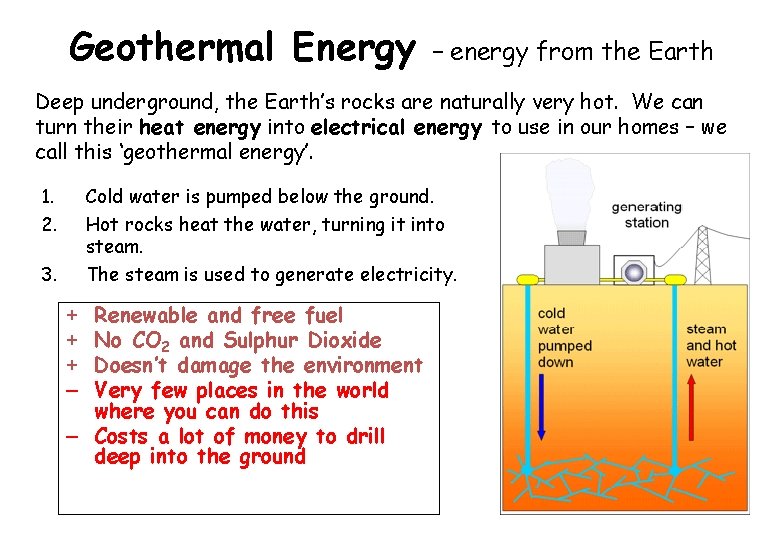
Geothermal Energy – energy from the Earth Deep underground, the Earth’s rocks are naturally very hot. We can turn their heat energy into electrical energy to use in our homes – we call this ‘geothermal energy’. 1. 2. Cold water is pumped below the ground. Hot rocks heat the water, turning it into steam. The steam is used to generate electricity. 3. Renewable and free fuel No CO 2 and Sulphur Dioxide Doesn’t damage the environment Very few places in the world where you can do this – Costs a lot of money to drill deep into the ground + + + –

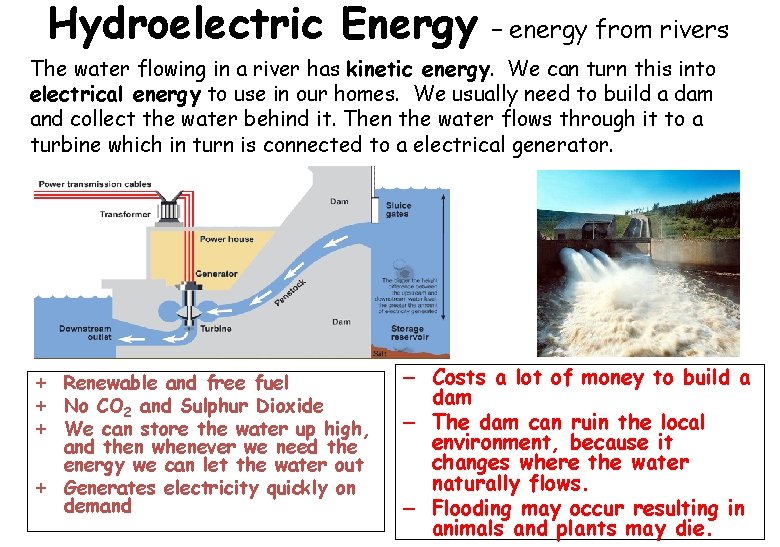
Hydroelectric Energy – energy from rivers The water flowing in a river has kinetic energy. We can turn this into electrical energy to use in our homes. We usually need to build a dam and collect the water behind it. Then the water flows through it to a turbine which in turn is connected to a electrical generator. + Renewable and free fuel + No CO 2 and Sulphur Dioxide + We can store the water up high, and then whenever we need the energy we can let the water out + Generates electricity quickly on demand – Costs a lot of money to build a dam – The dam can ruin the local environment, because it changes where the water naturally flows. – Flooding may occur resulting in animals and plants may die.

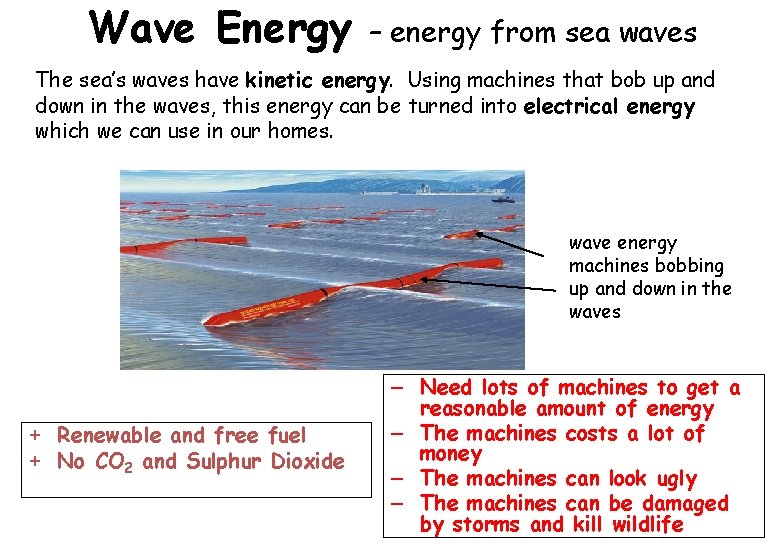
Wave Energy – energy from sea waves The sea’s waves have kinetic energy. Using machines that bob up and down in the waves, this energy can be turned into electrical energy which we can use in our homes. wave energy machines bobbing up and down in the waves + Renewable and free fuel + No CO 2 and Sulphur Dioxide – Need lots of machines to get a reasonable amount of energy – The machines costs a lot of money – The machines can look ugly – The machines can be damaged by storms and kill wildlife

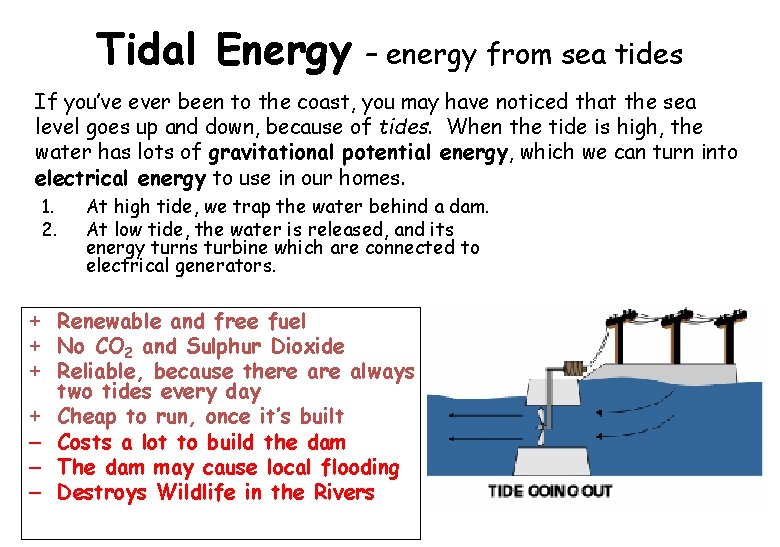
Tidal Energy – energy from sea tides If you’ve ever been to the coast, you may have noticed that the sea level goes up and down, because of tides. When the tide is high, the water has lots of gravitational potential energy, which we can turn into electrical energy to use in our homes. 1. 2. At high tide, we trap the water behind a dam. At low tide, the water is released, and its energy turns turbine which are connected to electrical generators. + Renewable and free fuel + No CO 2 and Sulphur Dioxide + Reliable, because there always two tides every day + Cheap to run, once it’s built – Costs a lot to build the dam – The dam may cause local flooding – Destroys Wildlife in the Rivers

Solar Energy – energy from the Sun The Earth gets heat and light energy from the sun all the time. Can we use it – yes we can! The Sun’s energy can either be: 1. Solar cells absorb the light energy from the sun and changed into electrical energy. 2. Solar panels can be used to heat water for homes. + Renewable – free fuel + No CO 2 and Sulphur Dioxide because nothing gets burned – Solar cells and solar panels are expensive – Only works if there is sunlight!

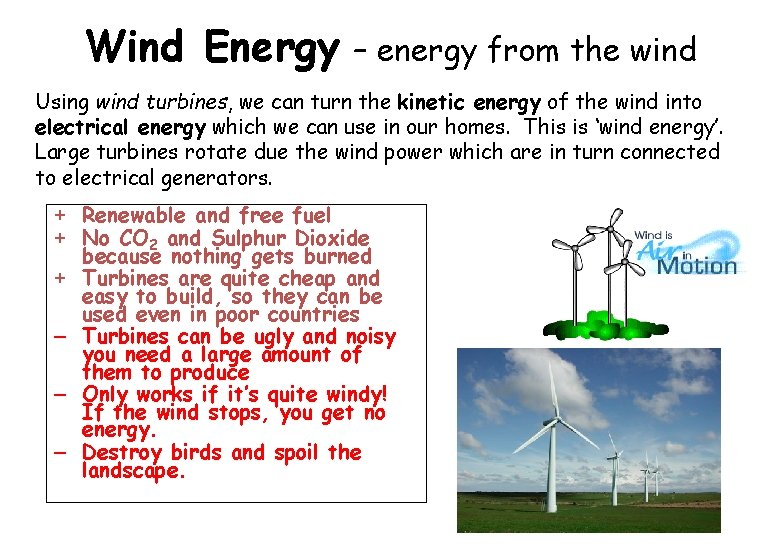
Wind Energy – energy from the wind Using wind turbines, we can turn the kinetic energy of the wind into electrical energy which we can use in our homes. This is ‘wind energy’. Large turbines rotate due the wind power which are in turn connected to electrical generators. + Renewable and free fuel + No CO 2 and Sulphur Dioxide because nothing gets burned + Turbines are quite cheap and easy to build, so they can be used even in poor countries – Turbines can be ugly and noisy you need a large amount of them to produce – Only works if it’s quite windy! If the wind stops, you get no energy. – Destroy birds and spoil the landscape.

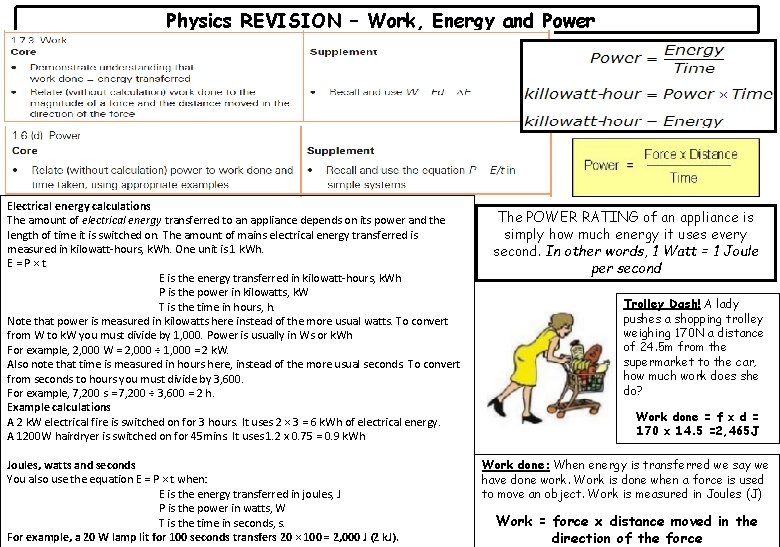
Physics REVISION – Work, Energy and Power Electrical energy calculations The amount of electrical energy transferred to an appliance depends on its power and the length of time it is switched on. The amount of mains electrical energy transferred is measured in kilowatt-hours, k. Wh. One unit is 1 k. Wh. E=P×t E is the energy transferred in kilowatt-hours, k. Wh P is the power in kilowatts, k. W T is the time in hours, h. Note that power is measured in kilowatts here instead of the more usual watts. To convert from W to k. W you must divide by 1, 000. Power is usually in Ws or k. Wh For example, 2, 000 W = 2, 000 ÷ 1, 000 = 2 k. W. Also note that time is measured in hours here, instead of the more usual seconds. To convert from seconds to hours you must divide by 3, 600. For example, 7, 200 s = 7, 200 ÷ 3, 600 = 2 h. Example calculations A 2 k. W electrical fire is switched on for 3 hours. It uses 2 × 3 = 6 k. Wh of electrical energy. A 1200 W hairdryer is switched on for 45 mins. It uses 1. 2 x 0. 75 = 0. 9 k. Wh Joules, watts and seconds You also use the equation E = P × t when: E is the energy transferred in joules, J P is the power in watts, W T is the time in seconds, s. For example, a 20 W lamp lit for 100 seconds transfers 20 × 100 = 2, 000 J (2 k. J). The POWER RATING of an appliance is simply how much energy it uses every second. In other words, 1 Watt = 1 Joule per second Trolley Dash! A lady pushes a shopping trolley weighing 170 N a distance of 24. 5 m from the supermarket to the car, how much work does she do? Work done = f x d = 170 x 14. 5 =2, 465 J Work done: When energy is transferred we say we have done work. Work is done when a force is used to move an object. Work is measured in Joules (J) Work = force x distance moved in the direction of the force

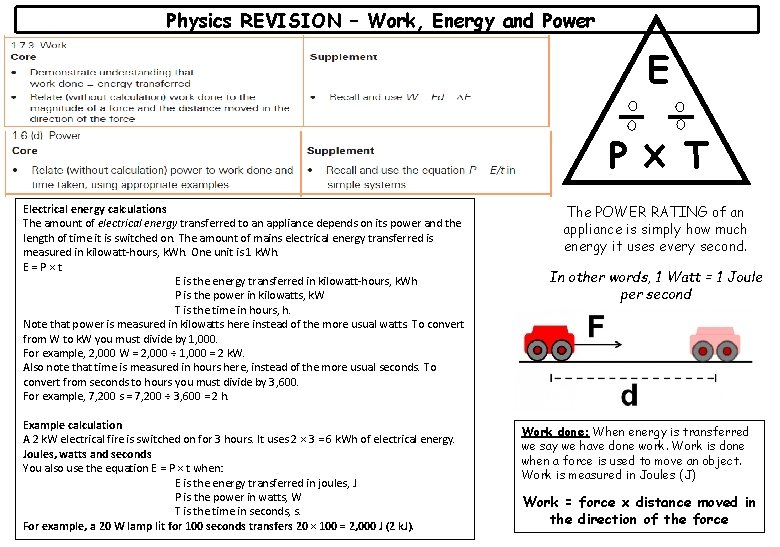
Physics REVISION – Work, Energy and Power E P Electrical energy calculations The amount of electrical energy transferred to an appliance depends on its power and the length of time it is switched on. The amount of mains electrical energy transferred is measured in kilowatt-hours, k. Wh. One unit is 1 k. Wh. E=P×t E is the energy transferred in kilowatt-hours, k. Wh P is the power in kilowatts, k. W T is the time in hours, h. Note that power is measured in kilowatts here instead of the more usual watts. To convert from W to k. W you must divide by 1, 000. For example, 2, 000 W = 2, 000 ÷ 1, 000 = 2 k. W. Also note that time is measured in hours here, instead of the more usual seconds. To convert from seconds to hours you must divide by 3, 600. For example, 7, 200 s = 7, 200 ÷ 3, 600 = 2 h. Example calculation A 2 k. W electrical fire is switched on for 3 hours. It uses 2 × 3 = 6 k. Wh of electrical energy. Joules, watts and seconds You also use the equation E = P × t when: E is the energy transferred in joules, J P is the power in watts, W T is the time in seconds, s. For example, a 20 W lamp lit for 100 seconds transfers 20 × 100 = 2, 000 J (2 k. J). T The POWER RATING of an appliance is simply how much energy it uses every second. In other words, 1 Watt = 1 Joule per second Work done: When energy is transferred we say we have done work. Work is done when a force is used to move an object. Work is measured in Joules (J) Work = force x distance moved in the direction of the force