Physics Revision Topic 3 Particle Model of Matter


- Slides: 17

Physics Revision Topic 3: Particle Model of Matter Miss Edwards


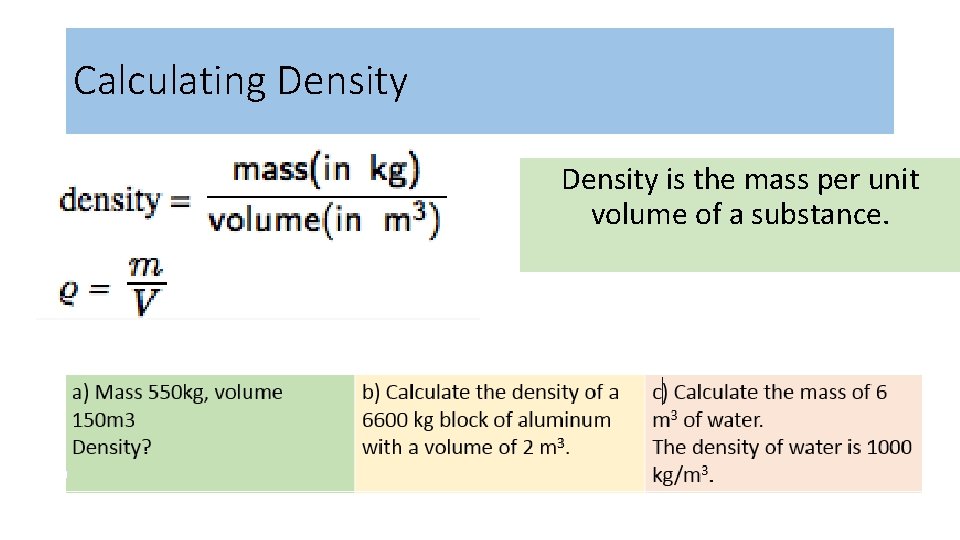
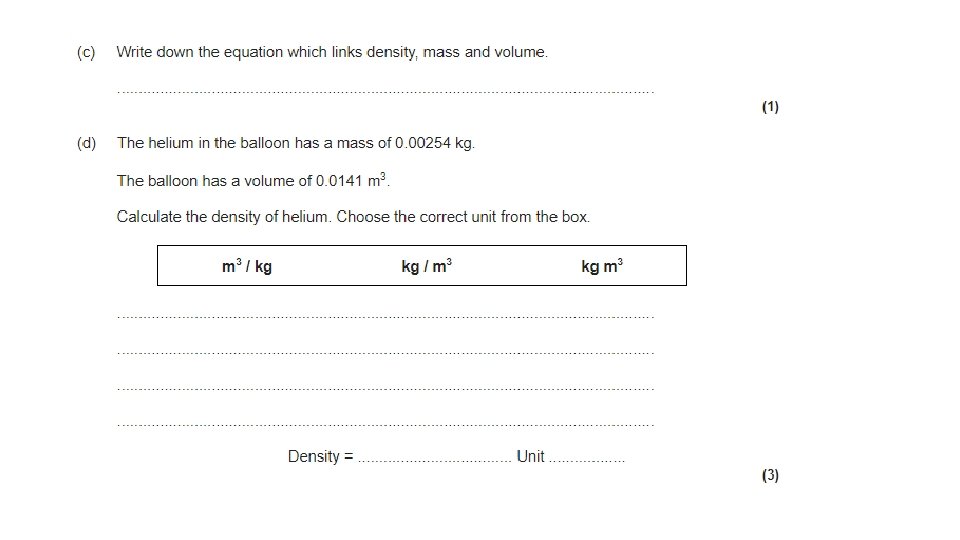
Calculating Density is the mass per unit volume of a substance.

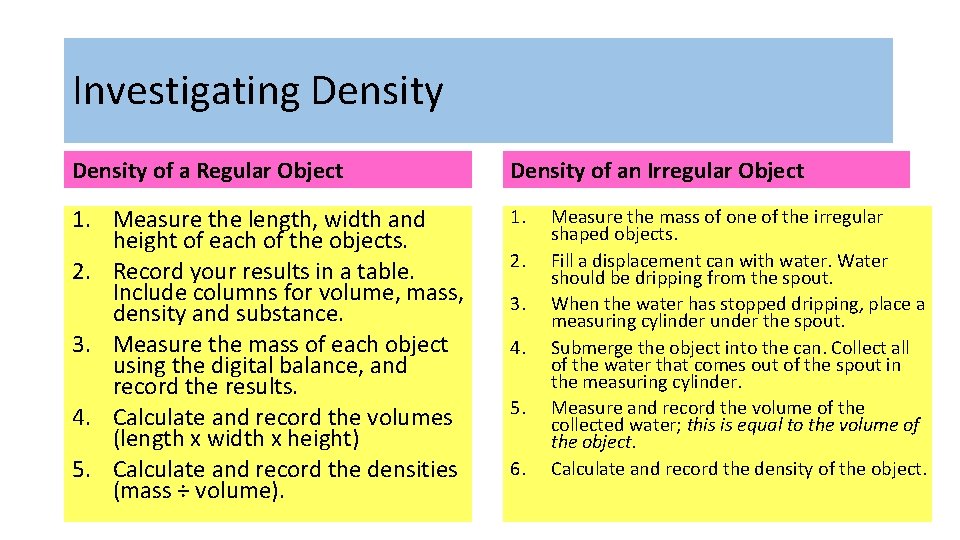
Investigating Density of a Regular Object Density of an Irregular Object 1. Measure the length, width and height of each of the objects. 2. Record your results in a table. Include columns for volume, mass, density and substance. 3. Measure the mass of each object using the digital balance, and record the results. 4. Calculate and record the volumes (length x width x height) 5. Calculate and record the densities (mass ÷ volume). 1. 2. 3. 4. 5. 6. Measure the mass of one of the irregular shaped objects. Fill a displacement can with water. Water should be dripping from the spout. When the water has stopped dripping, place a measuring cylinder under the spout. Submerge the object into the can. Collect all of the water that comes out of the spout in the measuring cylinder. Measure and record the volume of the collected water; this is equal to the volume of the object. Calculate and record the density of the object.

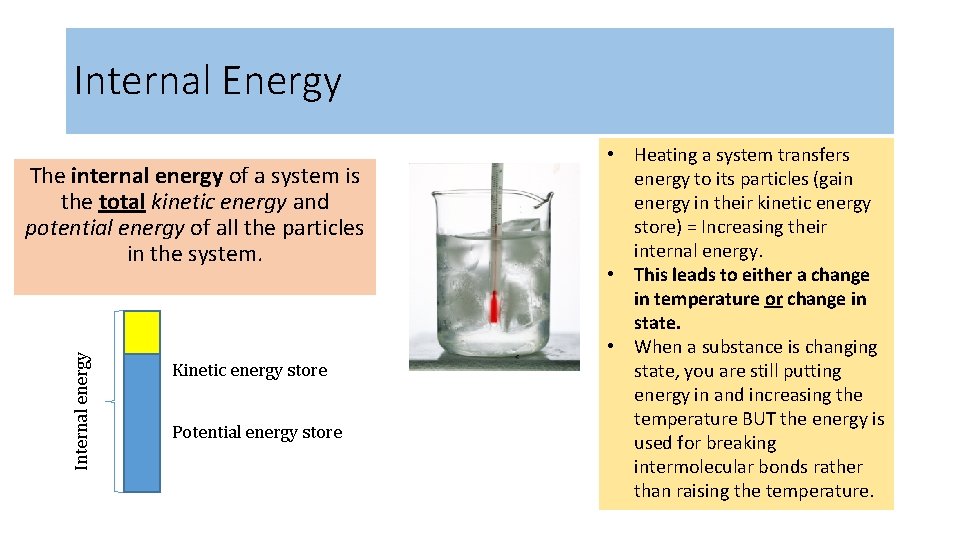
Internal Energy Internal energy The internal energy of a system is the total kinetic energy and potential energy of all the particles in the system. Kinetic energy store Potential energy store • Heating a system transfers energy to its particles (gain energy in their kinetic energy store) = Increasing their internal energy. • This leads to either a change in temperature or change in state. • When a substance is changing state, you are still putting energy in and increasing the temperature BUT the energy is used for breaking intermolecular bonds rather than raising the temperature.

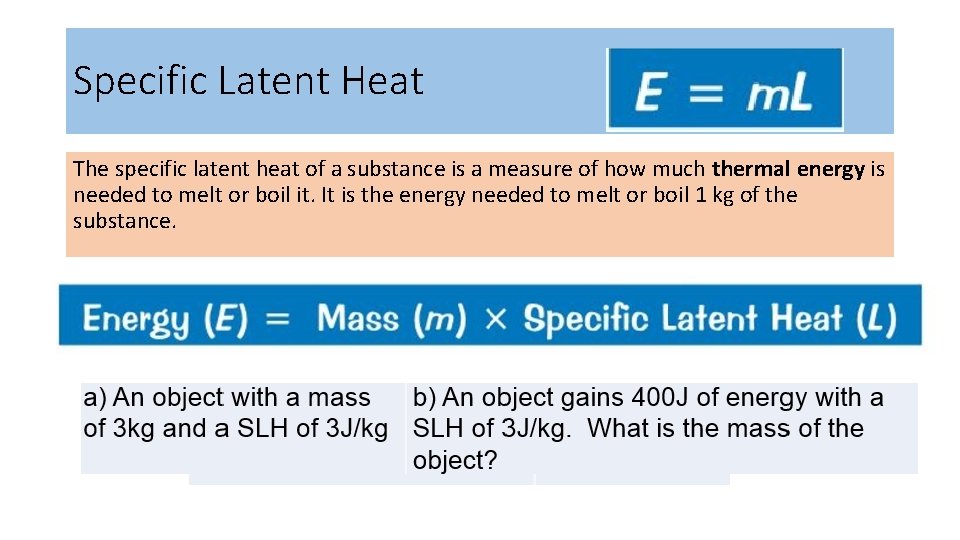
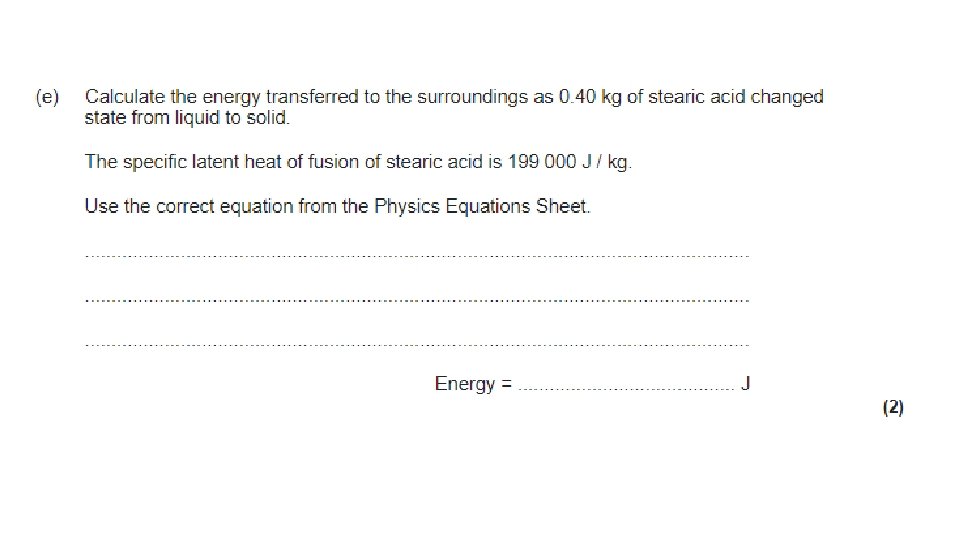
Specific Latent Heat The specific latent heat of a substance is a measure of how much thermal energy is needed to melt or boil it. It is the energy needed to melt or boil 1 kg of the substance.

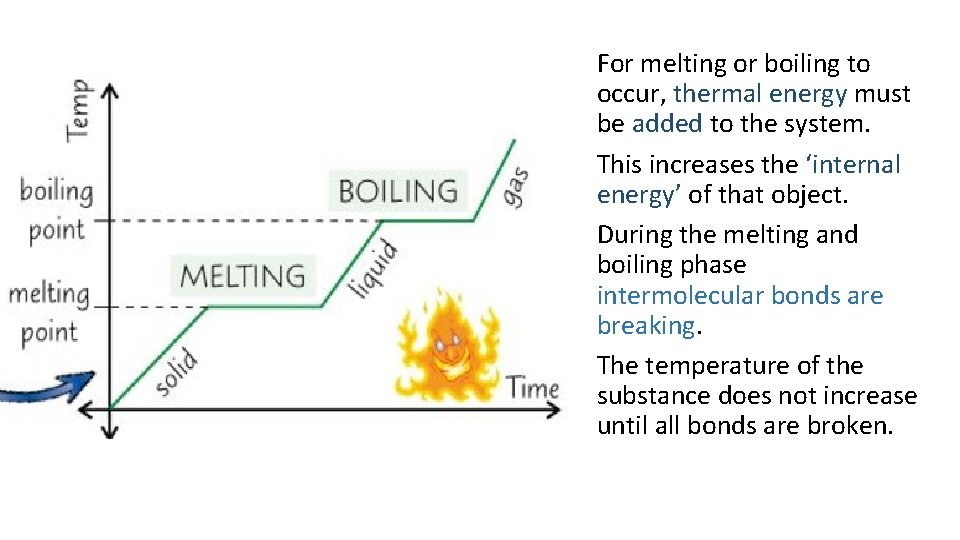
For melting or boiling to occur, thermal energy must be added to the system. This increases the ‘internal energy’ of that object. During the melting and boiling phase intermolecular bonds are breaking. The temperature of the substance does not increase until all bonds are broken.

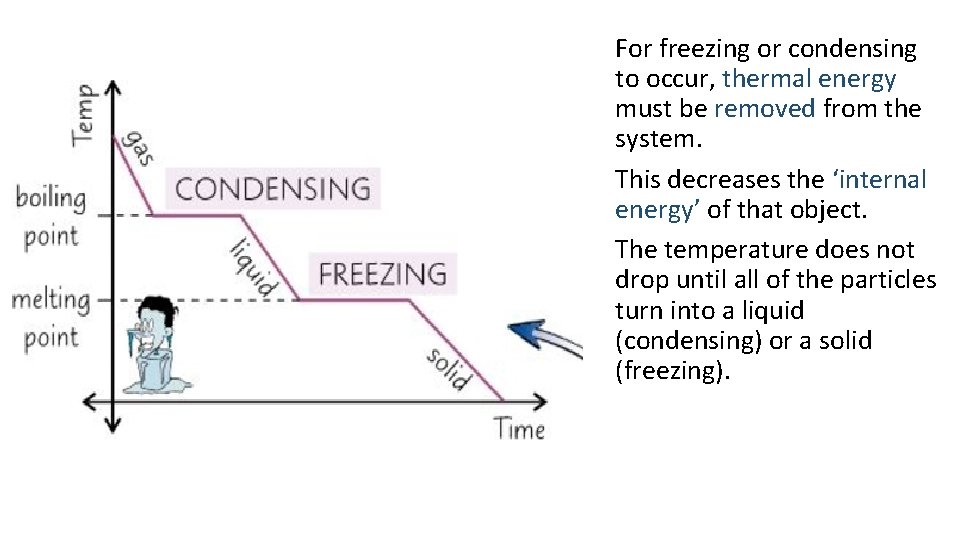
For freezing or condensing to occur, thermal energy must be removed from the system. This decreases the ‘internal energy’ of that object. The temperature does not drop until all of the particles turn into a liquid (condensing) or a solid (freezing).

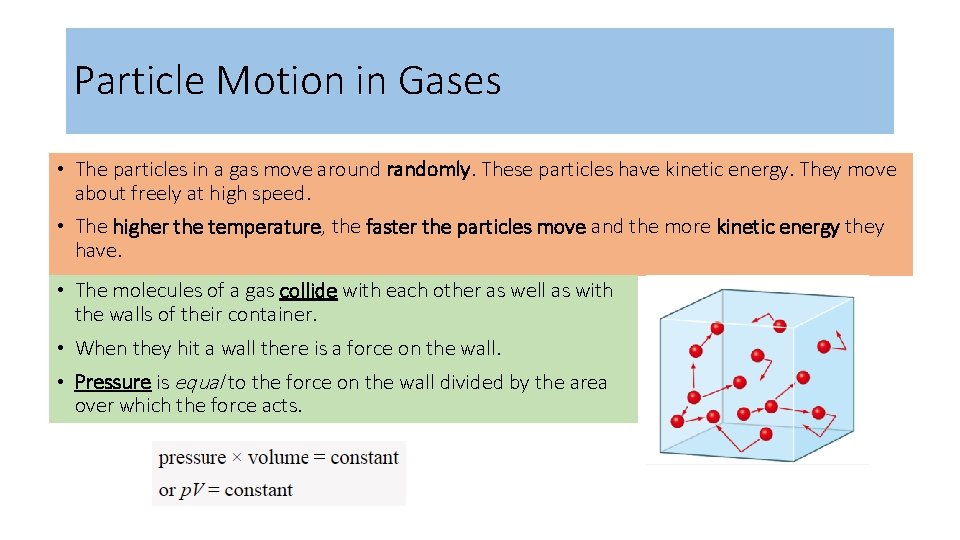
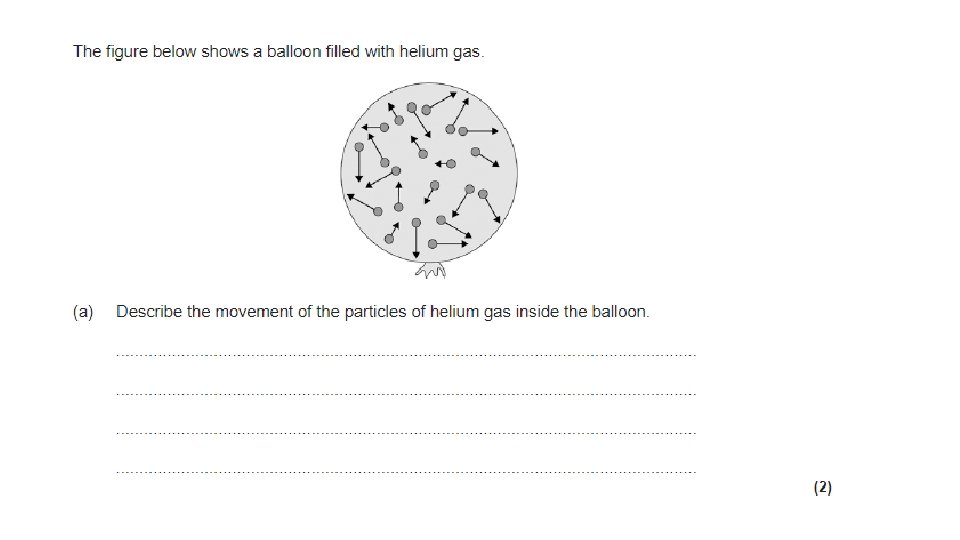
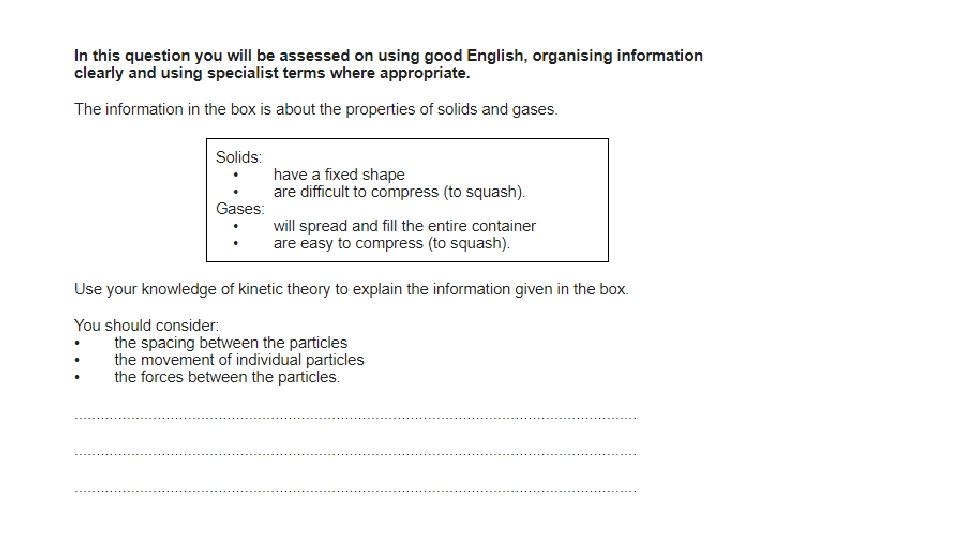
Particle Motion in Gases • The particles in a gas move around randomly. These particles have kinetic energy. They move about freely at high speed. • The higher the temperature, the faster the particles move and the more kinetic energy they have. • The molecules of a gas collide with each other as well as with the walls of their container. • When they hit a wall there is a force on the wall. • Pressure is equal to the force on the wall divided by the area over which the force acts.

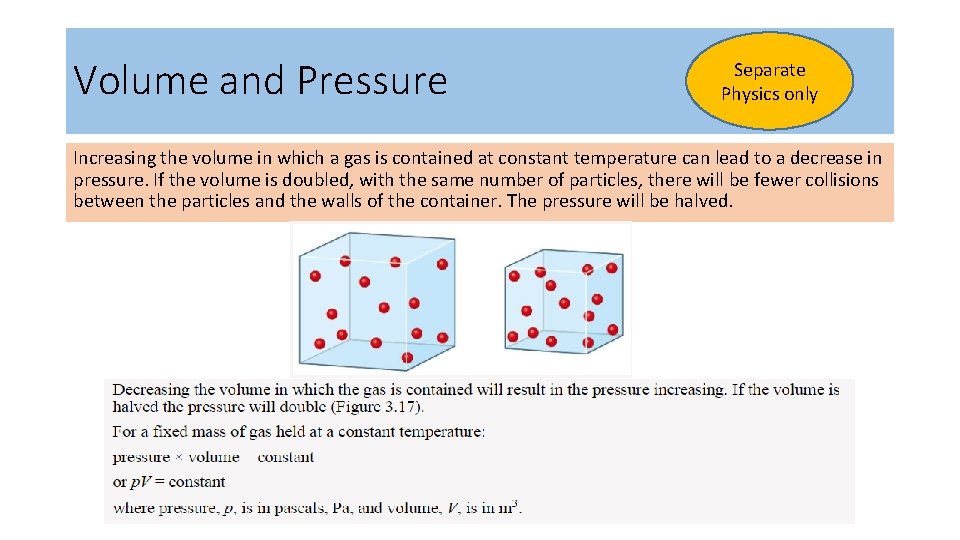
Volume and Pressure Separate Physics only Increasing the volume in which a gas is contained at constant temperature can lead to a decrease in pressure. If the volume is doubled, with the same number of particles, there will be fewer collisions between the particles and the walls of the container. The pressure will be halved.






