Physics of Technology PHYS 1800 Lecture 19 Introduction














- Slides: 32

Physics of Technology PHYS 1800 Lecture 19 Introduction Section 0 Fluids Lecture 1 Slide 1 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 1

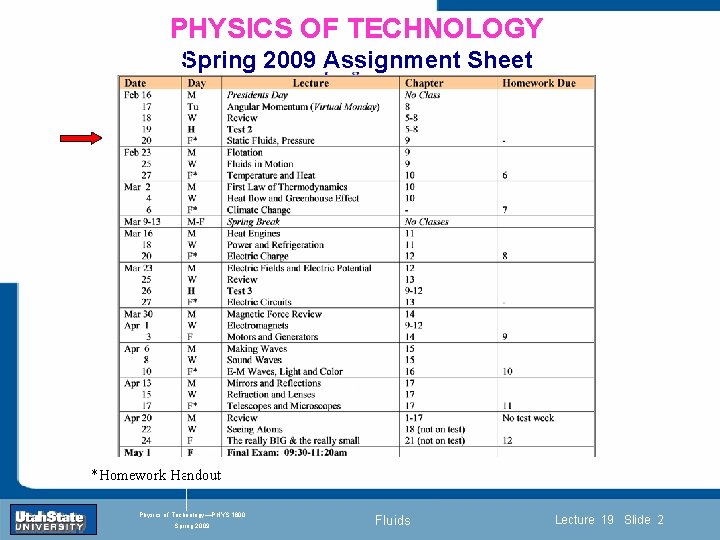
PHYSICS OF TECHNOLOGY Spring 2009 Assignment Sheet Introduction Section 0 Lecture 1 Slide 2 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 *Homework Handout Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 2

Physics of Technology PHYS 1800 Lecture 19 Fluids and Thermodynamics Introduction Section 0 Lecture 1 Slide 3 An Aside Into Atoms INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 3

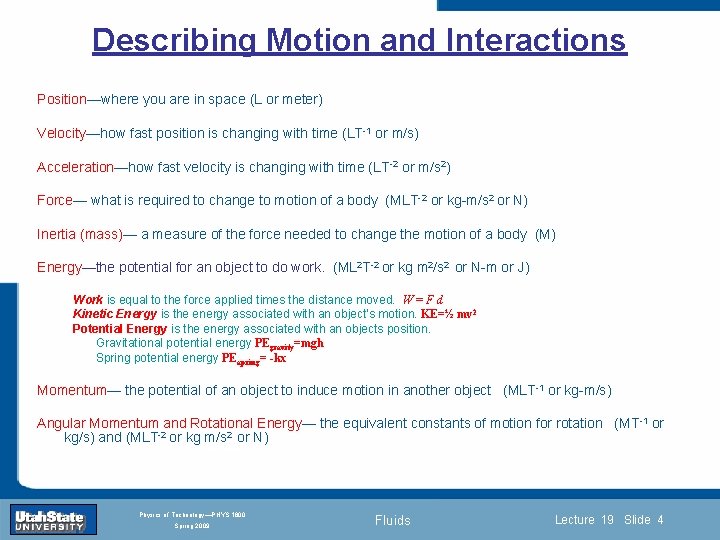
Describing Motion and Interactions Position—where you are in space (L or meter) Velocity—how fast position is changing with time (LT-1 or m/s) Acceleration—how fast velocity is changing with time (LT-2 or m/s 2) Force— what is required to change to motion of a body (MLT-2 or kg-m/s 2 or N) Inertia (mass)— a measure of the force needed to change the motion of a body (M) Energy—the potential for an object to do work. (ML 2 T-2 or kg m 2/s 2 or N-m or J) Work is equal to the force applied times the distance moved. W = F d Kinetic Energy is the energy associated with an object’s motion. KE=½ mv 2 Potential Energy is the energy associated with an objects position. Gravitational potential energy PEgravity=mgh Spring potential energy PEapring= -kx Lecture 1 Slide 4 in another object (MLT -1 or kg-m/s) Momentum—Introduction the potential. Section of an 0 object to induce motion Angular Momentum and Rotational Energy— the equivalent constants of motion for rotation (MT-1 or kg/s) and (MLT-2 or kg m/s 2 or N) INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 4

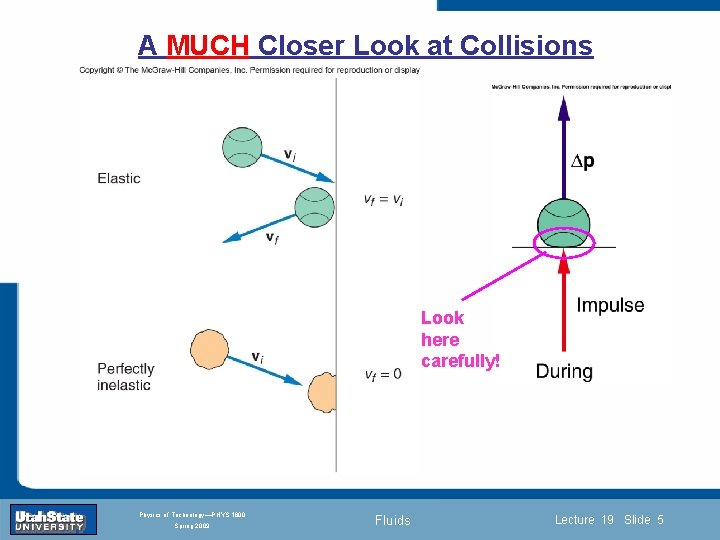
A MUCH Closer Look at Collisions Look here carefully! Introduction Section 0 Lecture 1 Slide 5 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 5

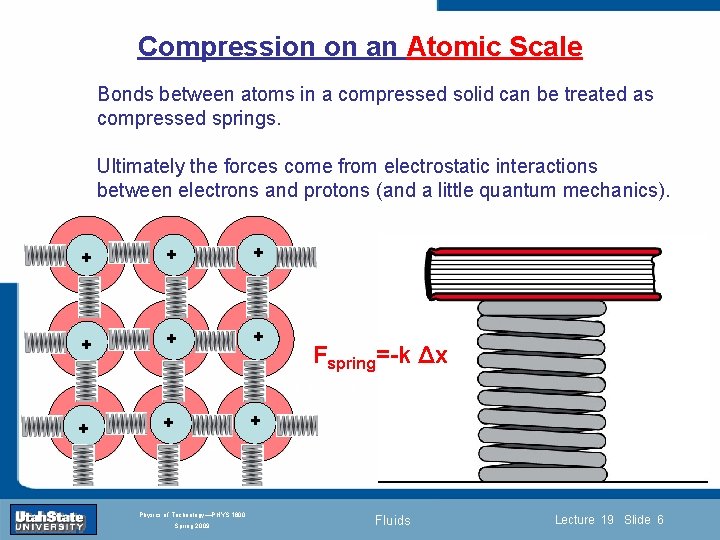
Compression on an Atomic Scale Bonds between atoms in a compressed solid can be treated as compressed springs. + + Ultimately the forces come from electrostatic interactions between electrons and protons (and a little quantum mechanics). + + Section 0 + Lecture 1 Slide 6 + + Introduction Fspring=-k Δx INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 6

Matter is made up of atoms… • The Atomic Theory, a cornerstone of modern science, was proposed by an early Greek thinker, Democritus (c. 460 BC - c. 370 BC). • 2400 year later, Feynman deemed this the most important notion in science Introduction Section 0 Lecture 1 Slide 7 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 7

So begins the quest to see atoms… • What is an atom? • What do atoms look like? • How do atoms move? • How big is an atom? Introduction Section 0 8 • Slide How can you see atoms? Lecture 1 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 8

The case for the existence of atoms… • Strong opposition to atomic theory: (1860 s) Lord Kelvin • The Periodic Table: (1871) Mendeleyev • Statistical Mechanics: (1890 s) Boltzmann • X-Rays: (1895) Röntgen • Quantum Theory: (1913) Bohr and Einstein Introduction Section 0 Lecture 1 Slide 9 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 9

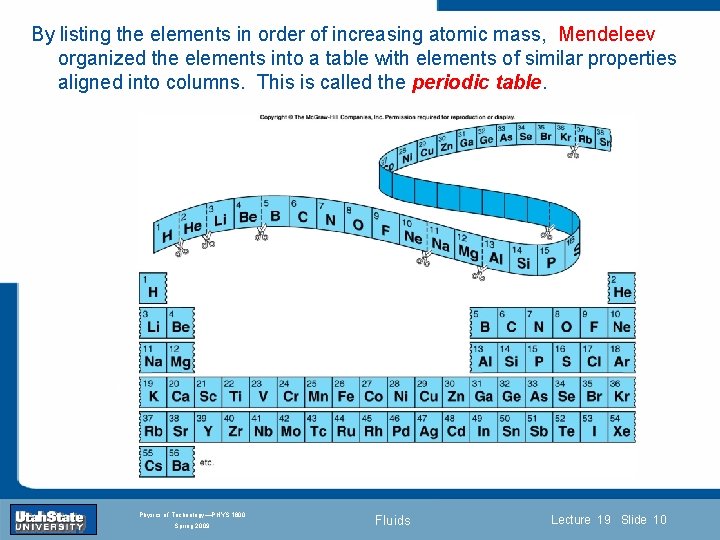
By listing the elements in order of increasing atomic mass, Mendeleev organized the elements into a table with elements of similar properties aligned into columns. This is called the periodic table. Introduction Section 0 Lecture 1 Slide 10 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 10

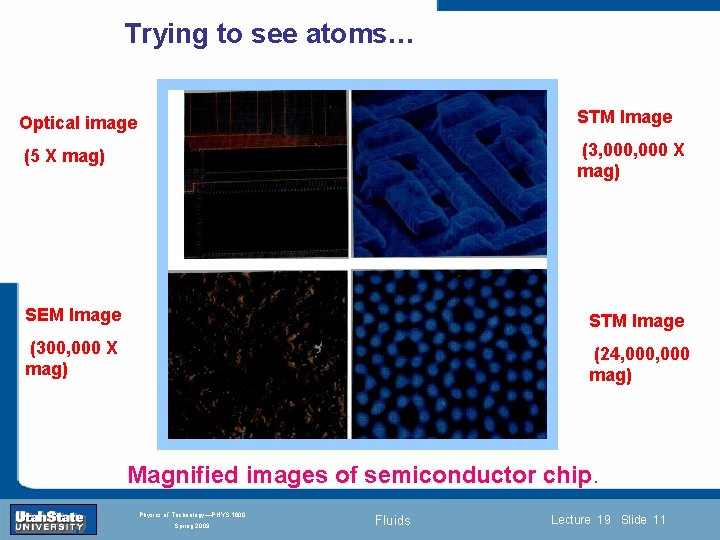
Trying to see atoms… Optical image STM Image (5 X mag) (3, 000 X mag) SEM Image STM Image (300, 000 X mag) (24, 000 mag) Introduction Section 0 Lecture 1 Slide 11 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Magnified images of semiconductor chip. Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 11

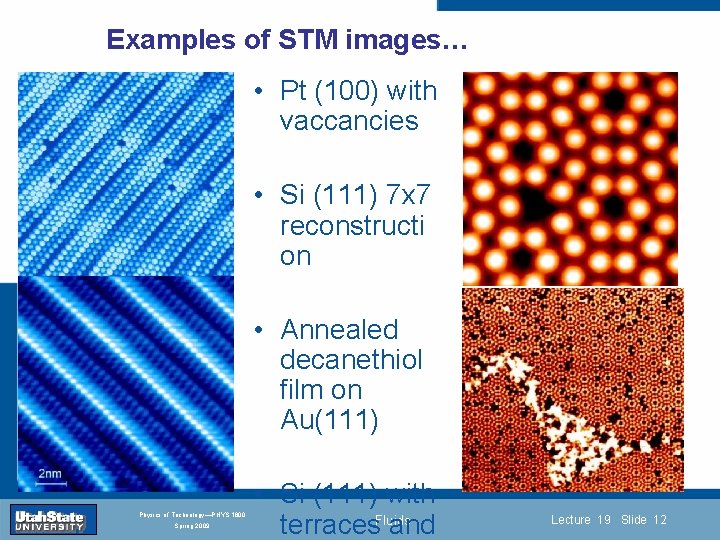
Examples of STM images… • Pt (100) with vaccancies • Si (111) 7 x 7 reconstructi on Introduction Section 0 • Annealed decanethiol Lecture 1 Slide 12 film on Au(111) INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 • Si (111) with terraces. Fluids and Lecture 19 Slide 12

Applications of knowledge on the atomic scale… • Feynman: “Plenty of room at the bottom” – Inevitability of small – Interface of quantum mechanics with applications Introduction Section 0 Lecture 1 Slide 13 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 13

Engineering Nanomachines Introduction Section 0 Lecture 1 Slide 15 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 15

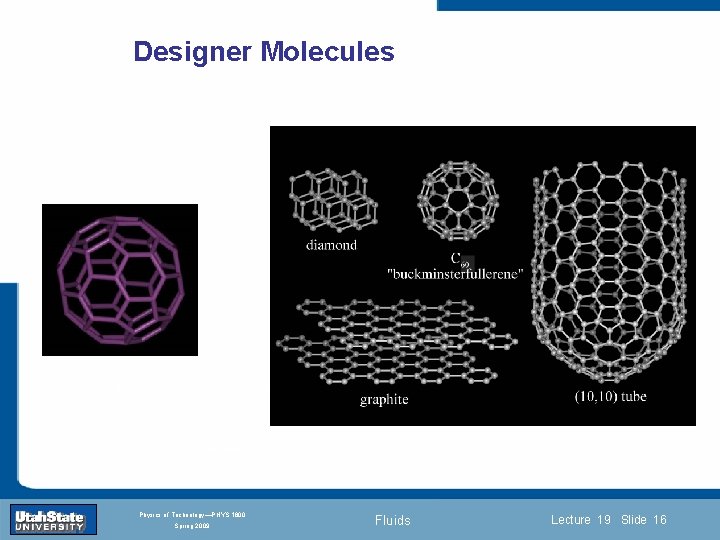
Designer Molecules Introduction Section 0 Lecture 1 Slide 16 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 16

Introduction Section 0 Lecture 1 Slide 17 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 17

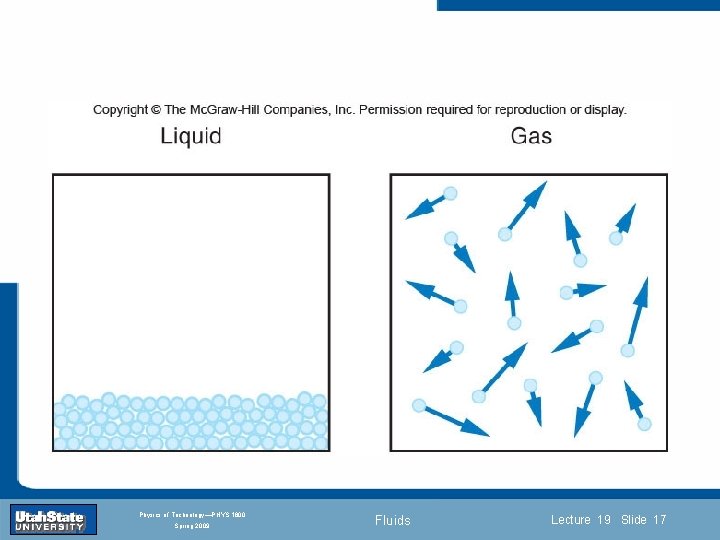
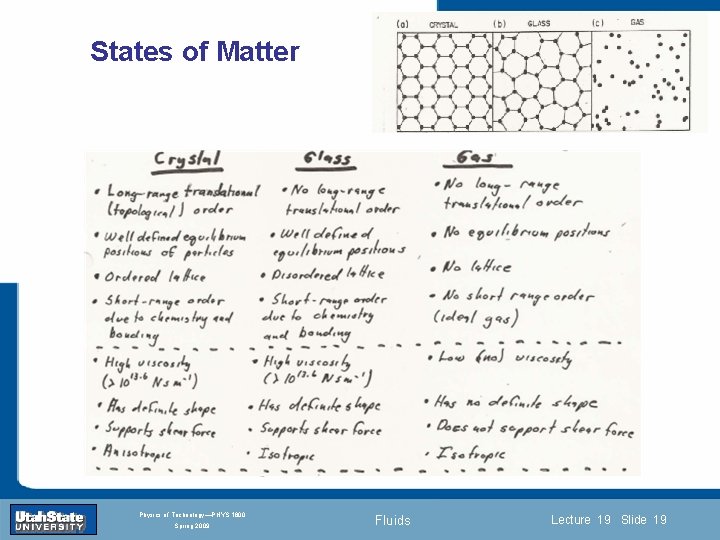
States of Matter Introduction Section 0 Lecture 1 Slide 18 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 18

States of Matter Introduction Section 0 Lecture 1 Slide 19 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 19

Understanding the behavior of fluids and UNIT TWO thermodynamics is Fluids and Heat crucial to understanding engines and energy utilization. Introduction Section 0 Lecture 1 Slide 20 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 20

UNIT TWO Fluids and Heat Introduction Section 0 Lecture 1 Slide 21 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 21

Physics of Technology PHYS 1800 Lecture 19 Fluids Introduction Section 0 Lecture 1 Slide 22 Introduction INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 22

Pressure explains. . . Introduction objects Section 0 Lecture 1 floating and moving fluids Slide 23 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 23

Dennison’s Laws of Fluids • When push comes to shove, fluids are just like other stuff. • Pascal’s Principle: Pressure extends uniformly in all directions in a fluid. • Boyle’s Law: Work on a fluid equals PΔV • Bernoulli’s Principle: Conservation of energy for fluids Introduction Section 0 Lecture 1 Slide 24 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 24

Physics of Technology PHYS 1800 Lecture 19 Fluids Introduction Pascal’s Lecture 1 Slide. Principle: 25 Pressure extends uniformly in all directions in a fluid. Section 0 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 25

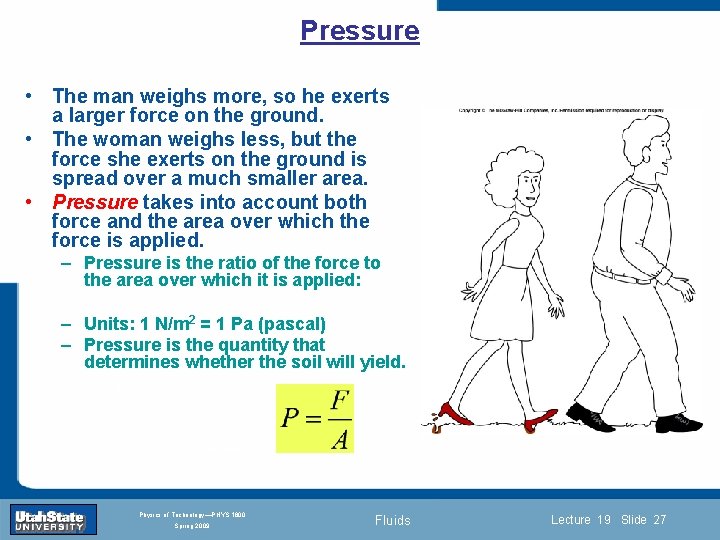
Pressure and Pascal’s Principle Why does a small woman wearing high-heel shoes sink into soft ground more than a large man wearing large shoes? Introduction Section 0 Lecture 1 Slide 26 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 26

Pressure • The man weighs more, so he exerts a larger force on the ground. • The woman weighs less, but the force she exerts on the ground is spread over a much smaller area. • Pressure takes into account both force and the area over which the force is applied. – Pressure is the ratio of the force to the area over which it is applied: – Units: 1 N/m 2 = 1 Pa (pascal) – Pressure is the quantity that determines whether the soil will yield. Introduction Section 0 Lecture 1 Slide 27 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 27

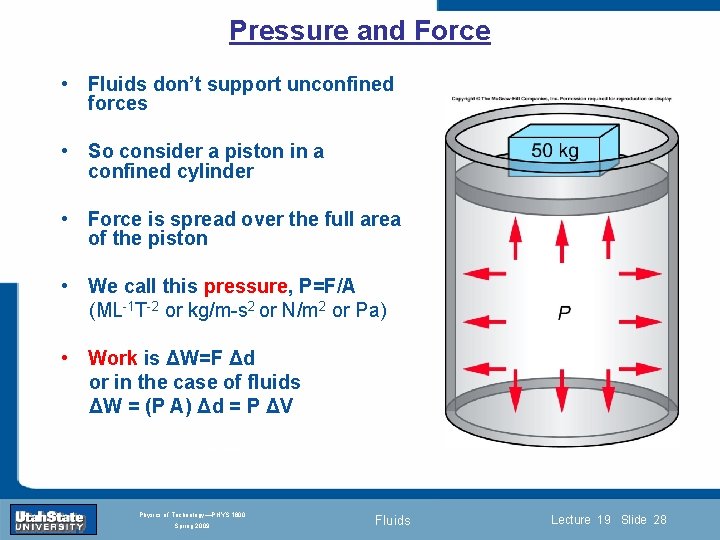
Pressure and Force • Fluids don’t support unconfined forces • So consider a piston in a confined cylinder • Force is spread over the full area of the piston • We call this pressure, P=F/A (ML-1 T-2 or kg/m-s 2 or N/m 2 or Pa) • Work is ΔW=F Δd or in the case of 0 fluids Introduction Section Lecture 1 ΔW = (P A) Δd = P ΔV Slide 28 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 28

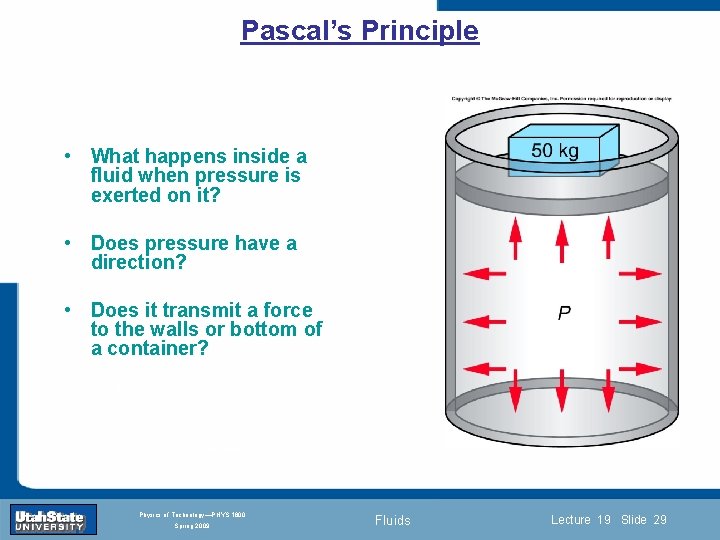
Pascal’s Principle • What happens inside a fluid when pressure is exerted on it? • Does pressure have a direction? • Does it transmit a force to the walls or bottom of a container? Introduction Section 0 Lecture 1 Slide 29 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 29

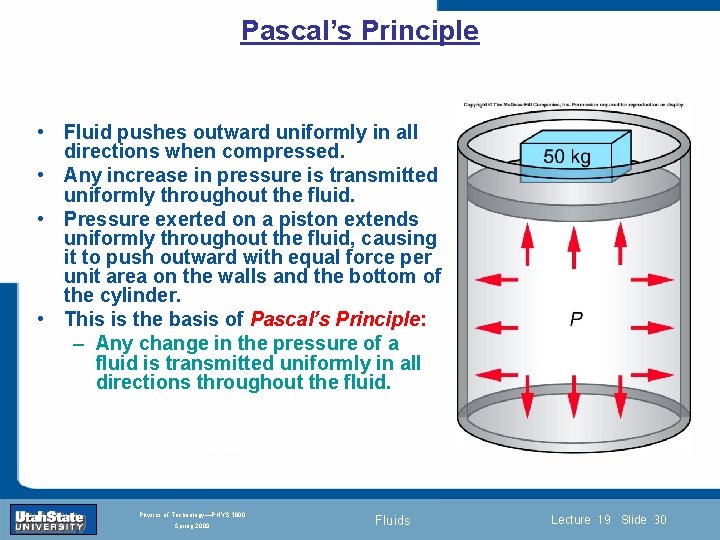
Pascal’s Principle • Fluid pushes outward uniformly in all directions when compressed. • Any increase in pressure is transmitted uniformly throughout the fluid. • Pressure exerted on a piston extends uniformly throughout the fluid, causing it to push outward with equal force per unit area on the walls and the bottom of the cylinder. • This is the basis of Pascal’s Principle: – Any change in the pressure of a fluid is transmitted uniformly in all directions throughout fluid. Introduction Section 0 Lecture 1 the Slide 30 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 30

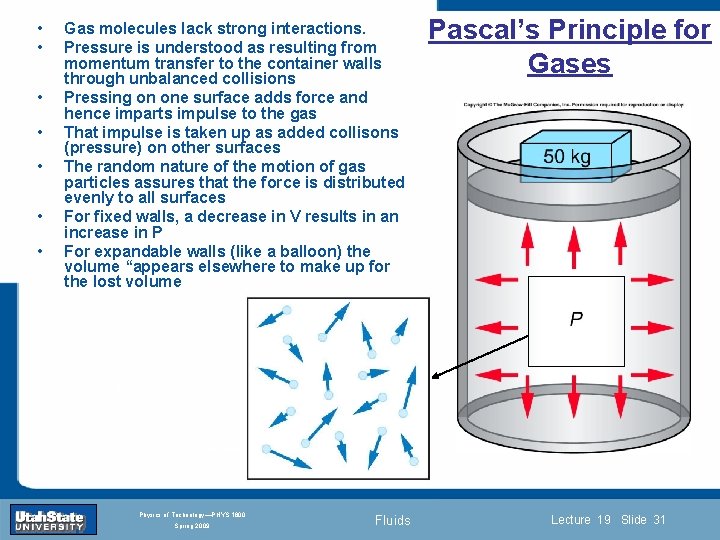
• • Gas molecules lack strong interactions. Pressure is understood as resulting from momentum transfer to the container walls through unbalanced collisions Pressing on one surface adds force and hence imparts impulse to the gas That impulse is taken up as added collisons (pressure) on other surfaces The random nature of the motion of gas particles assures that the force is distributed evenly to all surfaces For fixed walls, a decrease in V results in an increase in P For expandable walls (like a balloon) the volume “appears elsewhere to make up for the lost volume Introduction Section 0 Lecture 1 Pascal’s Principle for Gases Slide 31 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 31

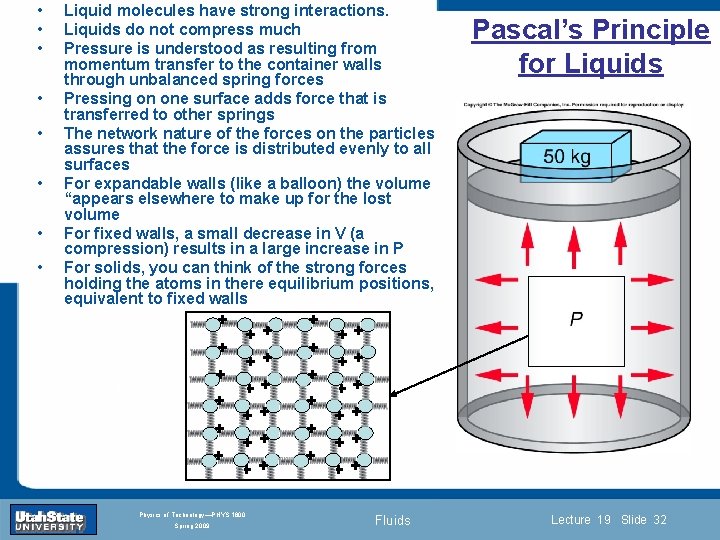
• • + Section 0 + Lecture 1 + + INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 + Physics of Technology—PHYS 1800 Spring 2009 Pascal’s Principle for Liquids + + + Introduction + + + + • Liquid molecules have strong interactions. Liquids do not compress much Pressure is understood as resulting from momentum transfer to the container walls through unbalanced spring forces Pressing on one surface adds force that is transferred to other springs The network nature of the forces on the particles assures that the force is distributed evenly to all surfaces For expandable walls (like a balloon) the volume “appears elsewhere to make up for the lost volume For fixed walls, a small decrease in V (a compression) results in a large increase in P For solids, you can think of the strong forces holding the atoms in there equilibrium positions, equivalent to fixed walls + + + • • • + + Slide + 32 + + + Fluids Lecture 19 Slide 32

Physics of Technology Next Lab/Demo: Rotational Motion Fluids Thursday 1: 30 -2: 45 ESLC 46 Ch 8 and 9 Next Class: Monday 10: 30 -11: 20 BUS Slide 33318 room Read Ch 9 Introduction Section 0 Lecture 1 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Fluids Lecture 19 Slide 33