Physics of ice Glaciers and ice sheets consist










- Slides: 14

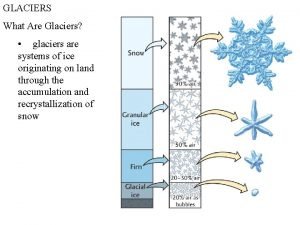
Physics of ice • Glaciers and ice sheets consist of ice – Thus the physical qualities of ice (and water) define the behaviour of these ice masses – The viscosity of ice is many orders of magnitude smaller than that of rocks – Ice and water coexist in glaciers, thus influence their dynamics

Water on Earth • 90%? in the interior of the Earth • 10% at or above the Earth’s surface, of which – 97. 390% in the oceans (salt water, sea ice) – 0. 019% in lakes and rivers (fresh water) – 0. 580% ground water – 2. 010% ice in ice sheets and glaciers – 0. 001% liquid, solis or gaseous in the atmosphere

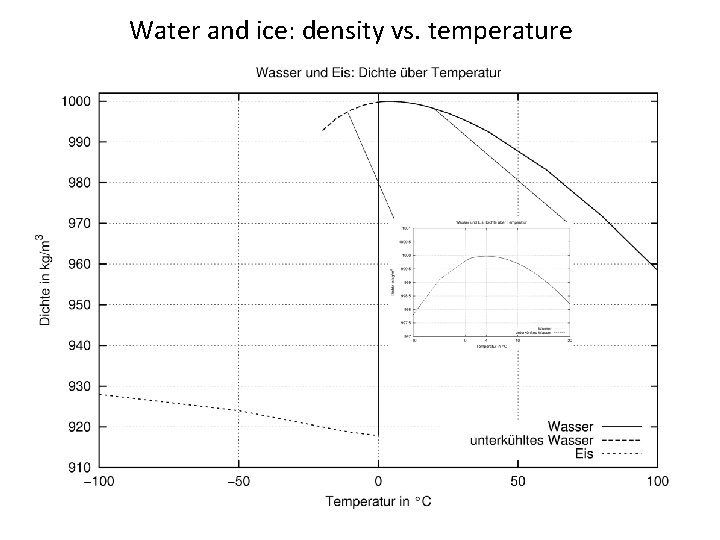
Water and Ice • Water (ice) is one of the most anomalous substances: The most important qualities: – – Ice floats on water Melting point decreases with increasing pressure Maximum density at 4 o. C Very high melting and freezing point compared to related compositions of related elements These qualities make life as we know possible!?

• High melting and boiling point: – Hydrogen bridges between the Oxigen atom of a water molecule and one of the Hydrogen atoms of the next

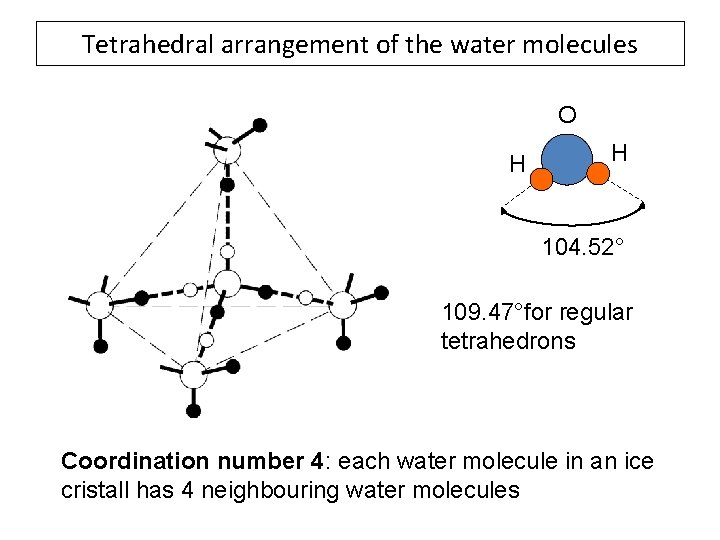
Tetrahedral arrangement of the water molecules O H H 104. 52° 109. 47°for regular tetrahedrons Coordination number 4: each water molecule in an ice cristall has 4 neighbouring water molecules

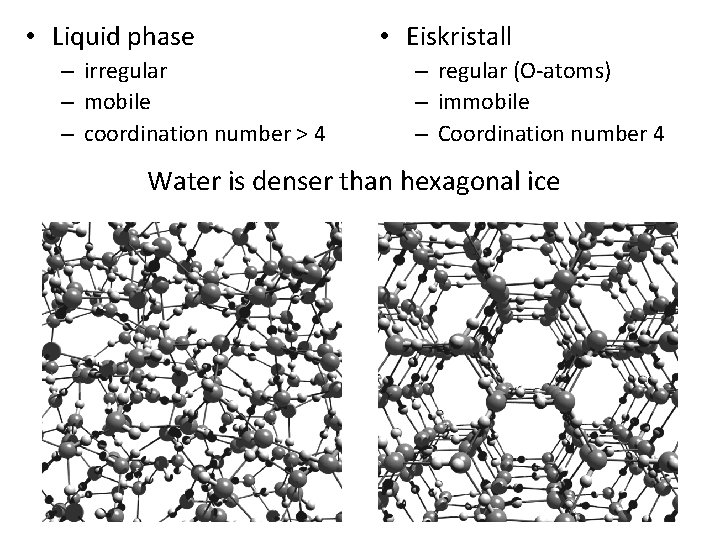
• Liquid phase – irregular – mobile – coordination number > 4 • Eiskristall – regular (O-atoms) – immobile – Coordination number 4 Water is denser than hexagonal ice

Water and ice: density vs. temperature

Annalen der Physik und Chemie, 1892, Vol. 43, S. 91 -97 heute nachgewiesen durch Streuexperimente mit den von Röntgen 1895 entdeckten „Röntgenstrahlen“. Er erhielt dafür den 1. Nobelpreis für Physik im Jahre 1901

Melting point of pure ice: pressure dependence Temperature relative to pressure melting point




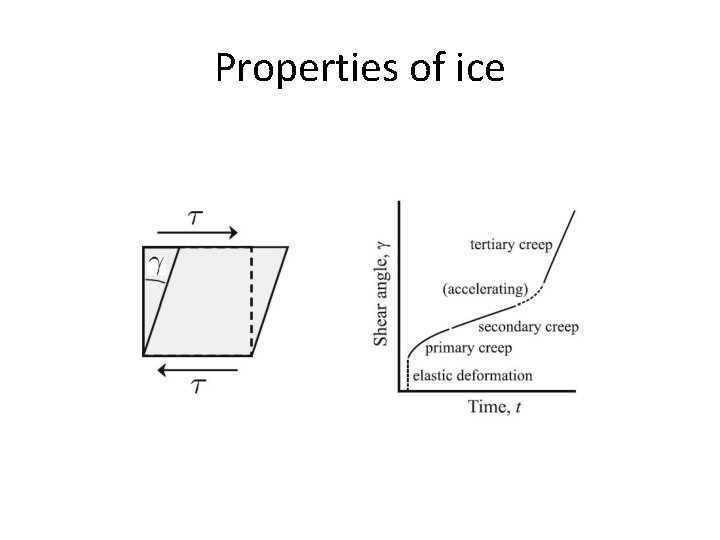
Properties of ice

 Glaciers cause erosion by abrasion and
Glaciers cause erosion by abrasion and Chapter 7 glaciers deserts and wind
Chapter 7 glaciers deserts and wind Examples of mass movement
Examples of mass movement Metamorphic rock
Metamorphic rock Moraine
Moraine Continental divide minnesota
Continental divide minnesota Alpine vs continental glaciers
Alpine vs continental glaciers What are the two main types of glaciers
What are the two main types of glaciers Is a valley
Is a valley Nsidc glaciers
Nsidc glaciers Bolivia glaciers
Bolivia glaciers Worksheet formulas consist of two components: operands and
Worksheet formulas consist of two components: operands and Is britain and uk same?
Is britain and uk same? What does the united kingdom consist of
What does the united kingdom consist of What does the united kingdom consist of
What does the united kingdom consist of