Physics of Fireworks Displays Basic Ingredients of Burning

Physics of Fireworks Displays

Basic Ingredients of Burning Fireworks 1. Oxidizing Agent (Nitrates, chlorates, perchlorates) – provides O 2 for burning e. g. potassium nitrate 2. Reducing Agent – burn the O 2 to produce hot gases, e. g. charcoal, sulfur 3. Coloring Agent – Salts that give color to the burn 4. Binder – binds the compound together, e. g. shellac • • Desire to slow down the burning of fuel Use larger grains of chemicals (250 -350 microns diameter)
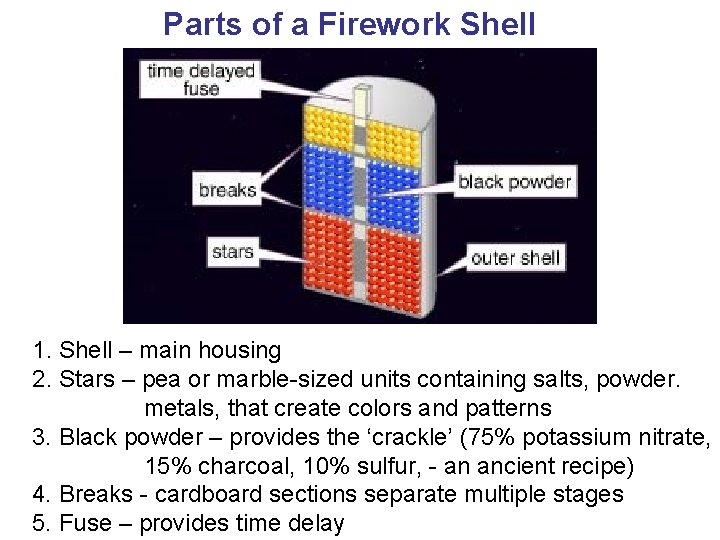
Parts of a Firework Shell 1. Shell – main housing 2. Stars – pea or marble-sized units containing salts, powder. metals, that create colors and patterns 3. Black powder – provides the ‘crackle’ (75% potassium nitrate, 15% charcoal, 10% sulfur, - an ancient recipe) 4. Breaks - cardboard sections separate multiple stages 5. Fuse – provides time delay

How is the firecracker shell fired ? • Shell is set in a steel or plastic mortar embedded in sand • An electronically ignited fuse sets off the charge • The hot gases from exploding powder propels the shell. • Time delay fuse needed to keep shell from exploding on the ground

Mortar Launch System for a Fireworks Display Time for delay fuse to burn determines pattern of explosion: 1. Spherical – explosion occurs at apex of parabolic trajectory. 2. Parabolic (or umbrella) -up – explosion occurs on the way up. 3. Parabolic (or umbrella) -down – explosion occurs on the way down
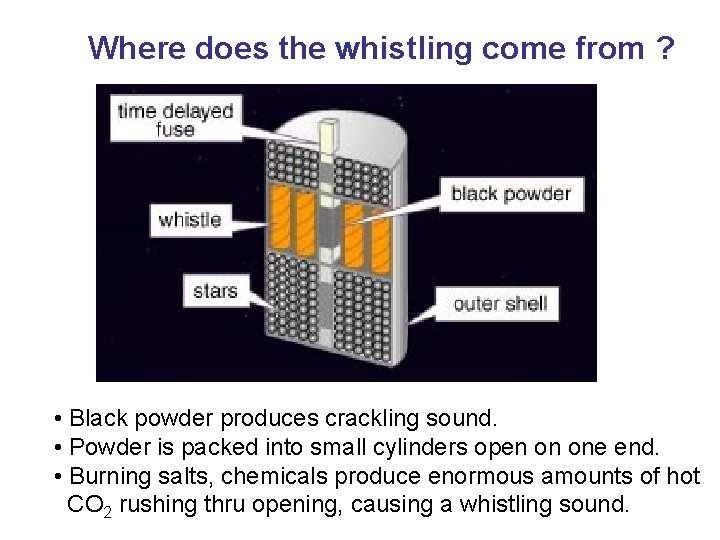
Where does the whistling come from ? • Black powder produces crackling sound. • Powder is packed into small cylinders open on one end. • Burning salts, chemicals produce enormous amounts of hot CO 2 rushing thru opening, causing a whistling sound.
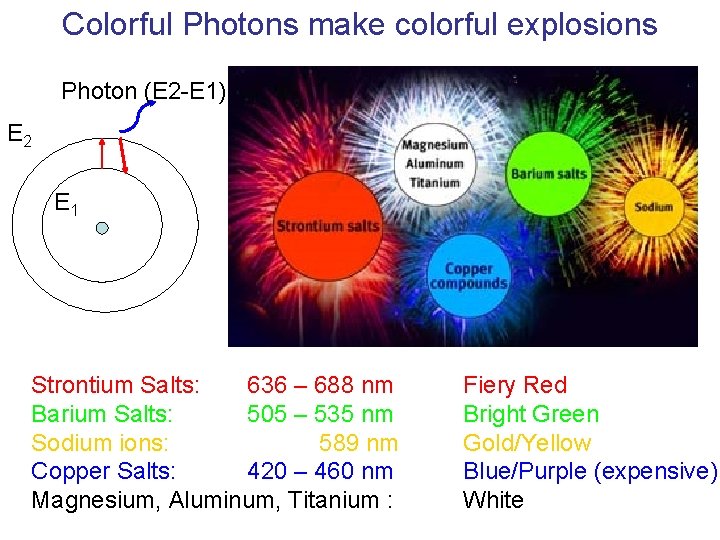
Colorful Photons make colorful explosions Photon (E 2 -E 1) E 2 E 1 Strontium Salts: 636 – 688 nm Barium Salts: 505 – 535 nm Sodium ions: 589 nm Copper Salts: 420 – 460 nm Magnesium, Aluminum, Titanium : Fiery Red Bright Green Gold/Yellow Blue/Purple (expensive) White

Examples of exploding Salts Shell exploding on the way up causes an ‘umbrella’ (really parabolic) pattern of burning salts.
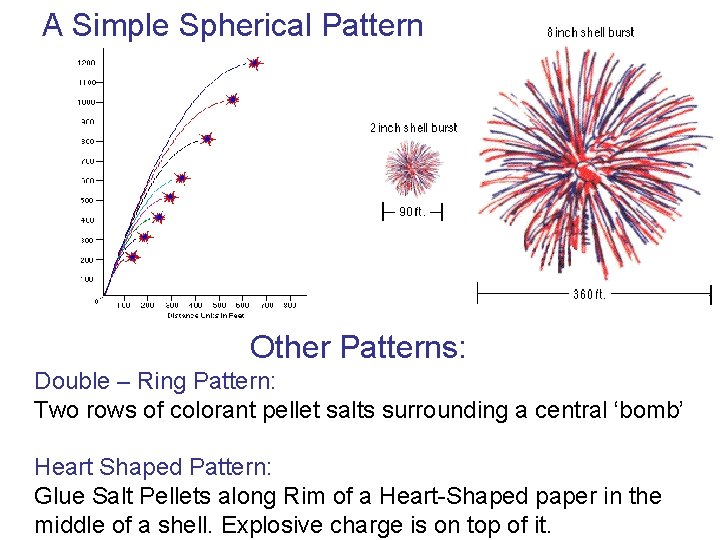
A Simple Spherical Pattern Other Patterns: Double – Ring Pattern: Two rows of colorant pellet salts surrounding a central ‘bomb’ Heart Shaped Pattern: Glue Salt Pellets along Rim of a Heart-Shaped paper in the middle of a shell. Explosive charge is on top of it.
- Slides: 9