Physics and the Quantum Mechanical Model Light Waves




- Slides: 10

Physics and the Quantum Mechanical Model

Light (Waves) • Amplitude – The waves height from zero to the crest • Wavelength (λ) – The distance between two crests. • Frequency (ν) – The number of waves that pass a given point per unit time. Usually expressed in Hertz (Hz), cycles per second (s-1).

Light Waves (Continued) • c=λν The product of frequency and wavelength always equals a constant (c), the speed of light (2. 998 x 108 m/s ) What type of relationship does wavelength and frequency have? (hint: linear, inverse, or quadratic) Inverse. If we rearrange the formula to solve for wavelength, we get the following equation: λ=c/ν What happens to wavelength as frequency increases? Wavelength decreases

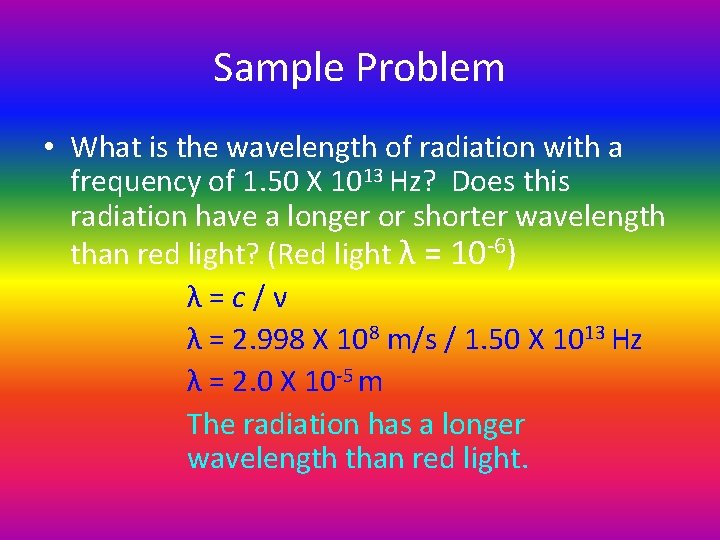
Sample Problem • What is the wavelength of radiation with a frequency of 1. 50 X 1013 Hz? Does this radiation have a longer or shorter wavelength than red light? (Red light λ = 10 -6) λ=c/ν λ = 2. 998 X 108 m/s / 1. 50 X 1013 Hz λ = 2. 0 X 10 -5 m The radiation has a longer wavelength than red light.

Light Waves (Continued) • Light consists of electromagnetic waves • Names a few types of electromagnetic radiation: – Radio waves, microwaves, infrared waves, visible light, ultraviolet waves. X-rays, and gamma rays. • All electromagnetic waves travel in a vacuum at a speed of 2. 998 X 108 m/s speed of light

Light Waves (Continued) • What happens when sunlight passes through a prism? – Different frequencies separate into a spectrum of colors. (Atomic emission spectrum) • Name a natural phenomenon where light get separated into its constituent spectrum of colors. – A rainbow

Atomic Spectra • Passing an electric current through a gas in a neon tube energizes the electrons of the atoms of gas, and causes them to emit light. • When atoms absorb energy, electrons move to higher energy levels, and these electrons lose energy by emitting light when they return to lower energy levels.

Atomic Spectra Explanation • Ground state - The lowest possible energy level of an electron. Here the principal quantum number (n) is 1. – Excitation of an electron raises it from the ground state to an excited state (n) = 2, 3, 4, 5, etc… – The light emitted by the electron moving from a higher to a lower energy level has a frequency directly proportional to the energy of the electron

Quantum Mechanics • Describes the motions of subatomic particles and atoms as waves. • Light quanta – Photon • Heisenberg uncertainty principal – It is impossible to know exactly both the velocity and the position of a particle at the same time.
