Physics 2130 Foundations of Modern Physics Quantum Mechanics








- Slides: 42

Physics 2130 Foundations of Modern Physics Quantum Mechanics Part IV: Applications Clicker Questions Lecture Slides Professor John Price, Spring 2019

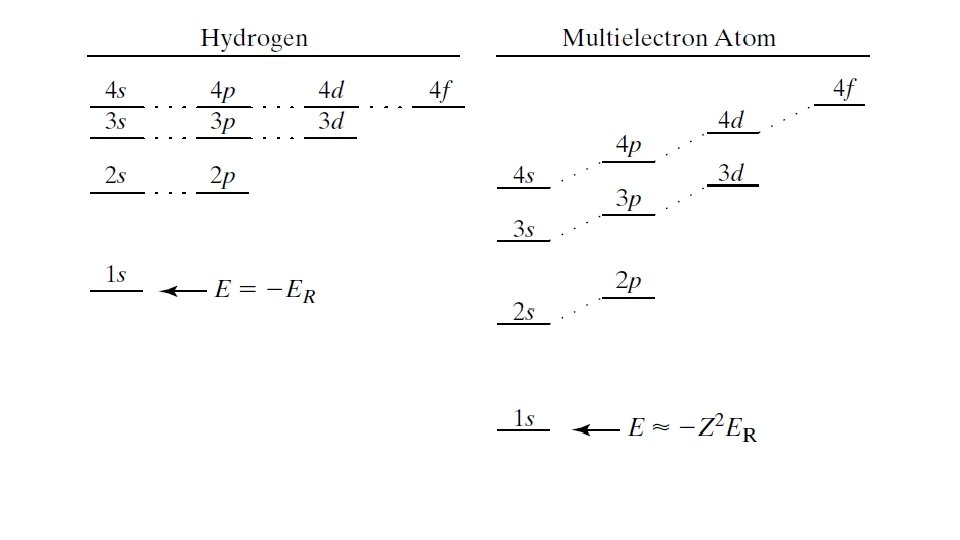
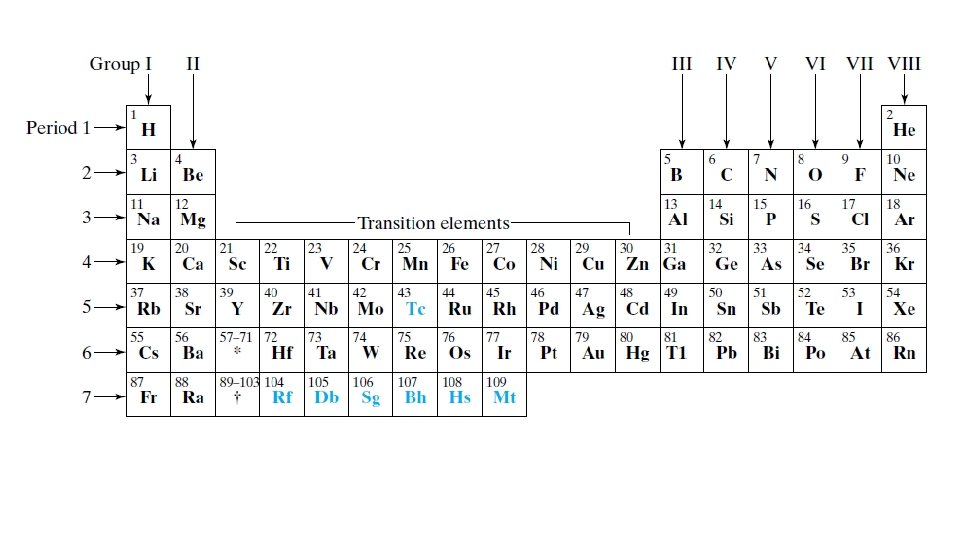
Applications of Quantum Mechanics Multi-electron atoms and the periodic table (TZD Ch. 10) Metals, insulators, semiconductors (TZD 13. 1 - 13. 6, 14. 2) Nuclear spin, NMR, MRI (TZD 16. 1, 16. 2, 9. 3, 9. 4, 9. 5, 9. 8) Quantum Computing J. Preskill: https: //arxiv. org/abs/1801. 00862 A. Helwer on Youtube: https: //youtu. be/F_Riqjdh 2 o. M 2

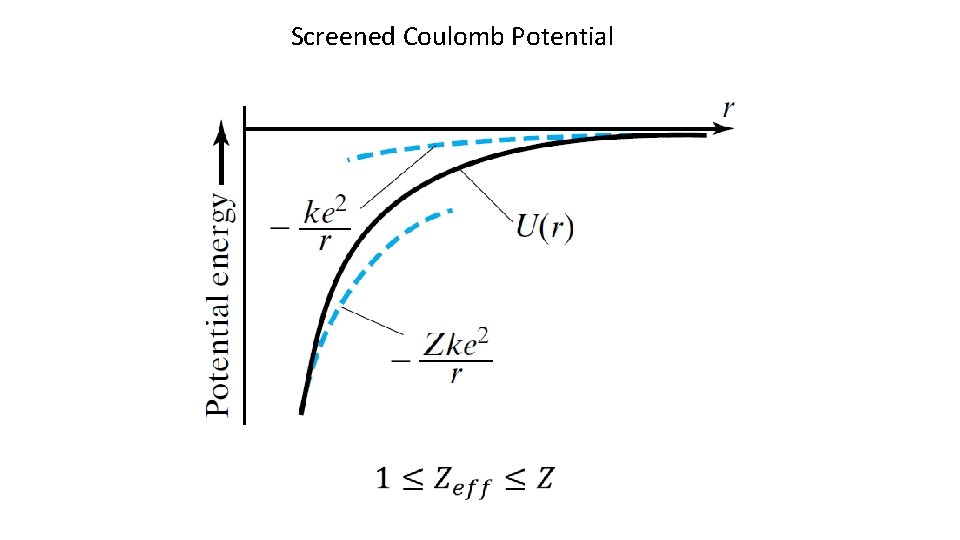
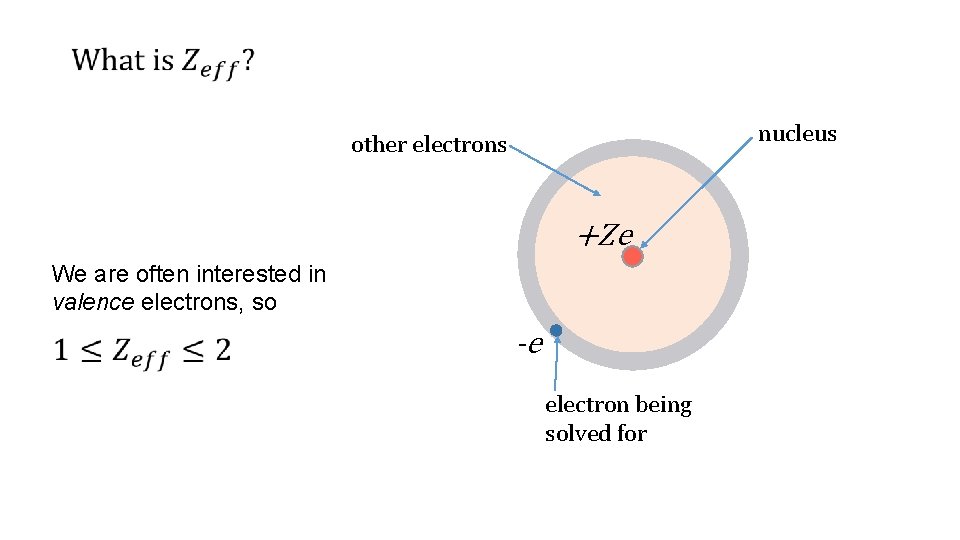
Screened Coulomb Potential


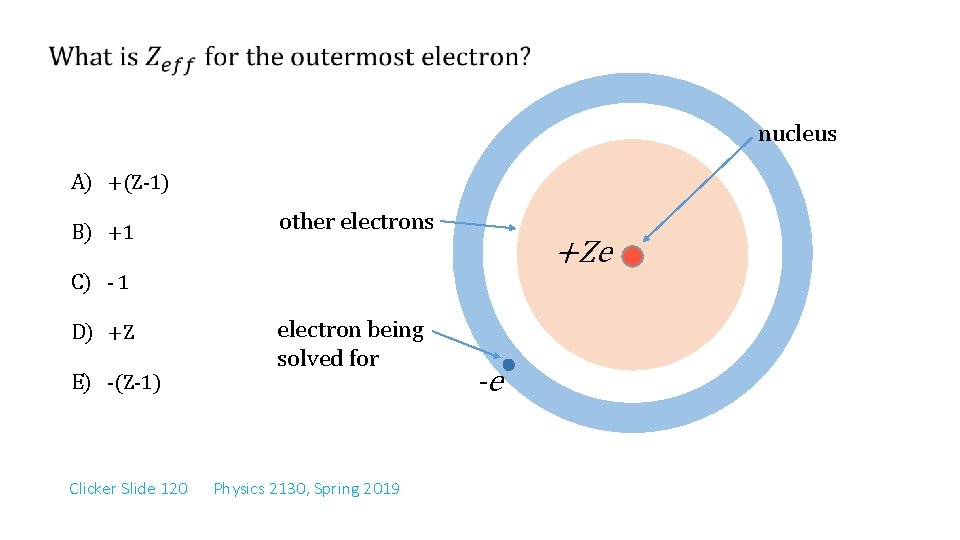
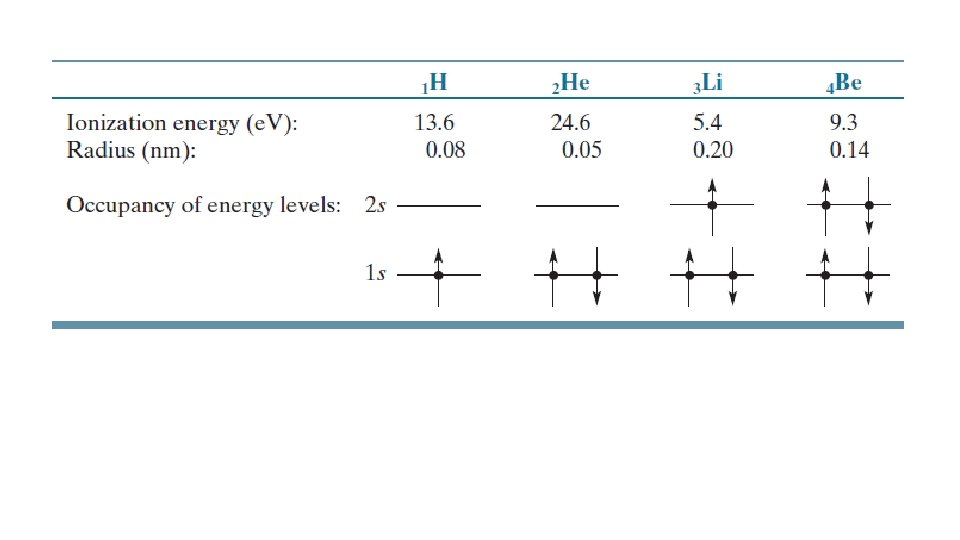
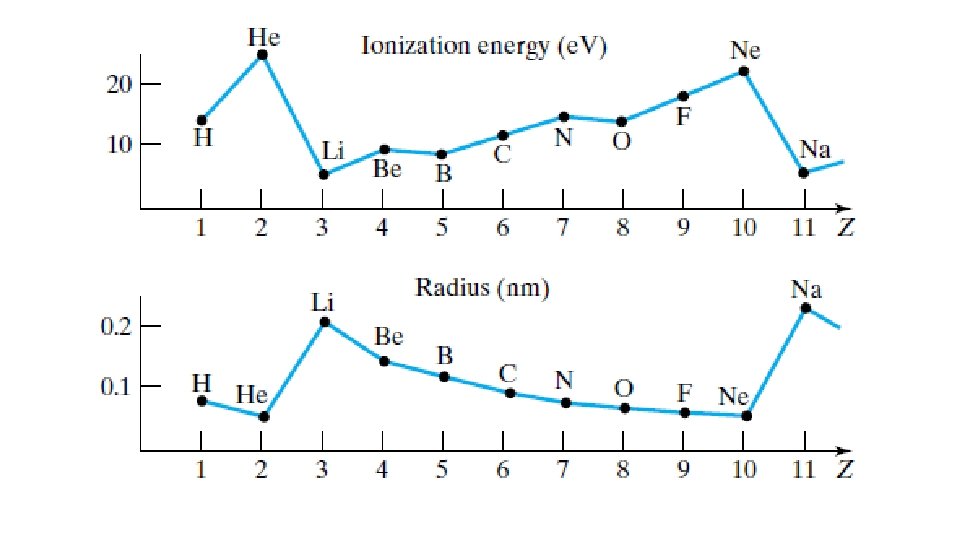
nucleus A) +(Z-1) B) +1 other electrons +Ze C) - 1 D) +Z electron being solved for E) -(Z-1) Clicker Slide 120 Physics 2130, Spring 2019 -e

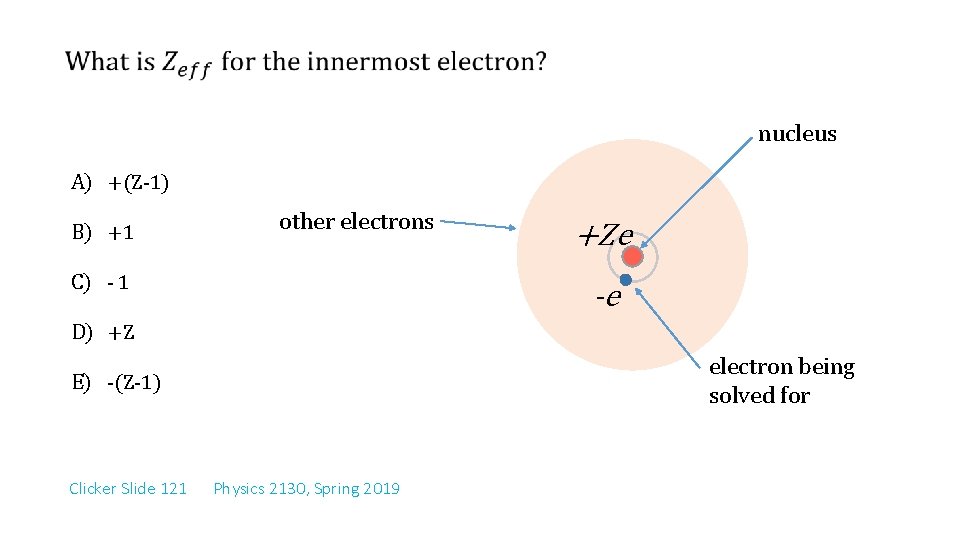
nucleus A) +(Z-1) B) +1 other electrons C) - 1 +Ze -e D) +Z electron being solved for E) -(Z-1) Clicker Slide 121 Physics 2130, Spring 2019

nucleus other electrons +Ze We are often interested in valence electrons, so -e electron being solved for



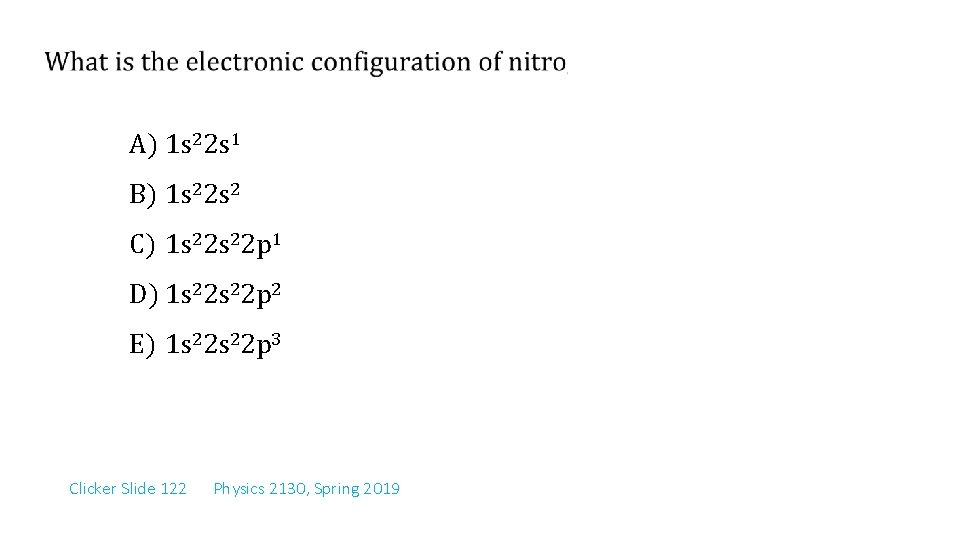
A) 1 s 22 s 1 B) 1 s 22 s 2 C) 1 s 22 p 1 D) 1 s 22 p 2 E) 1 s 22 p 3 Clicker Slide 122 Physics 2130, Spring 2019


A) 1. 0 B) 1. 4 C) 2. 0 D) 2. 4 Clicker Slide 123 Physics 2130, Spring 2019


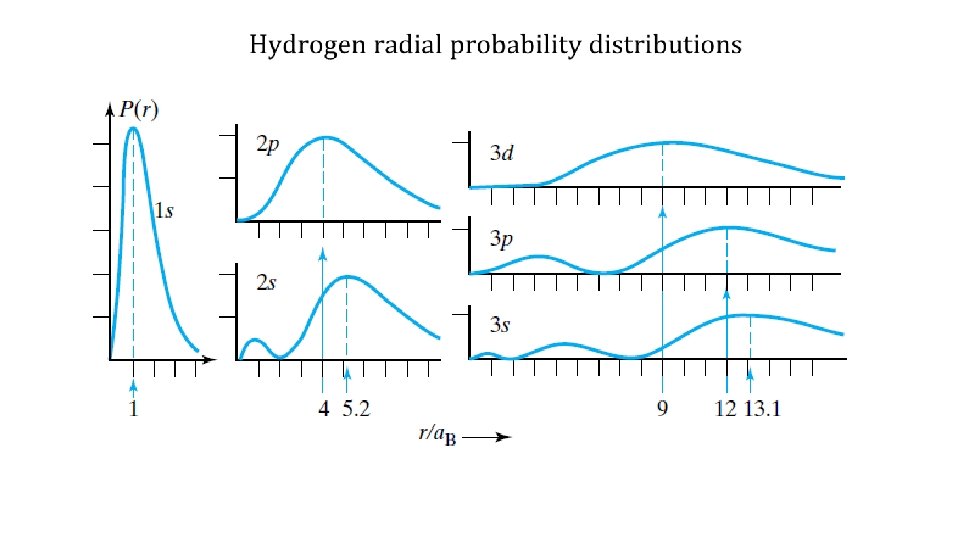
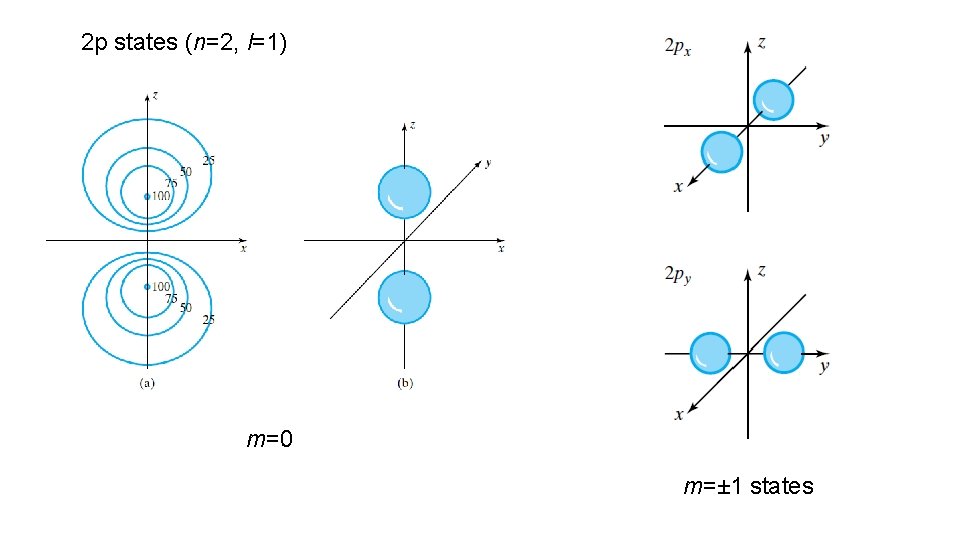
2 p states (n=2, l=1) m=0 m=± 1 states

Crystals Ca. SO 4 2 H 2 O Blue: Ca Red: Oxygen Yellow: Sulfer Selenite crystals, Niaca Mine, Chihuahua Pink: Oxygen in H 2 O

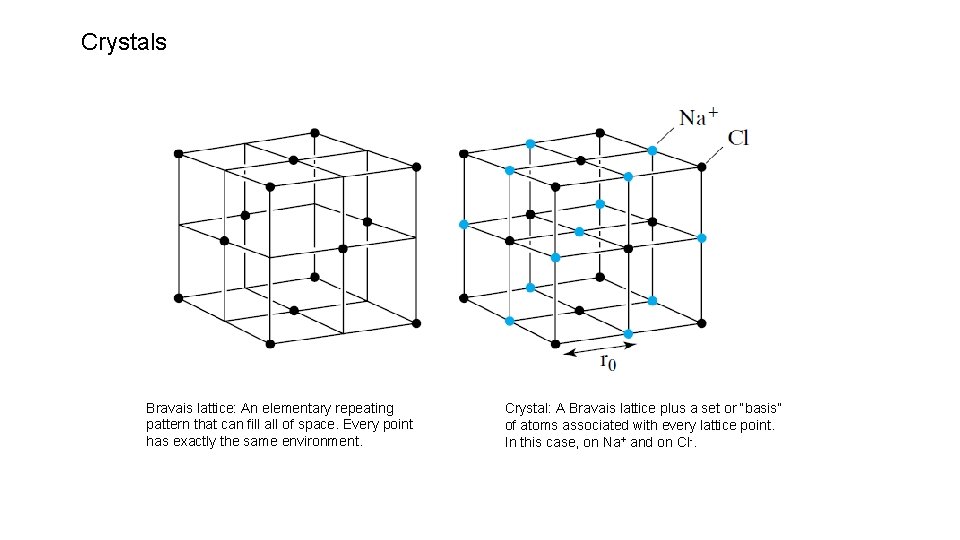
Crystals Bravais lattice: An elementary repeating pattern that can fill all of space. Every point has exactly the same environment. Crystal: A Bravais lattice plus a set or “basis” of atoms associated with every lattice point. In this case, on Na+ and on Cl-.

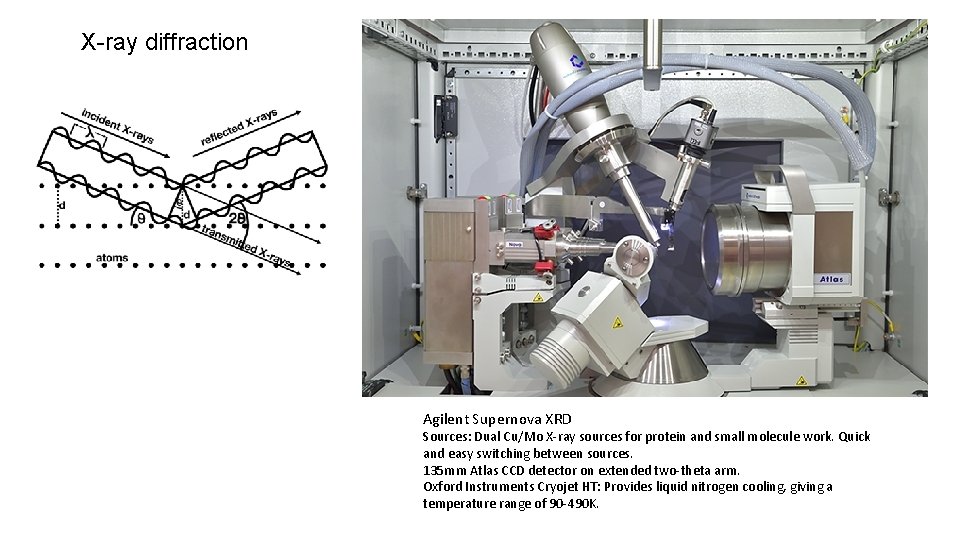
X-ray diffraction Agilent Supernova XRD Sources: Dual Cu/Mo X-ray sources for protein and small molecule work. Quick and easy switching between sources. 135 mm Atlas CCD detector on extended two-theta arm. Oxford Instruments Cryojet HT: Provides liquid nitrogen cooling, giving a temperature range of 90 -490 K.

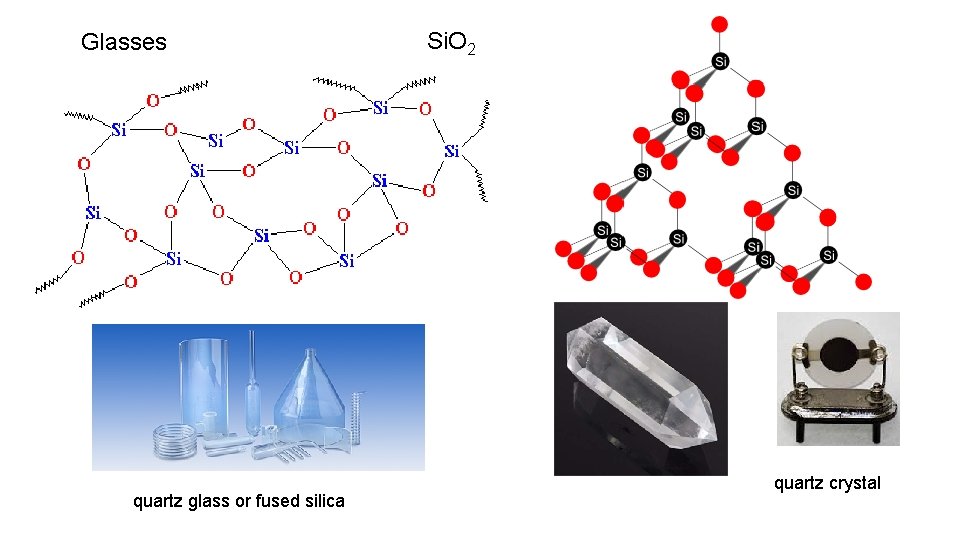
Glasses quartz glass or fused silica Si. O 2 quartz crystal

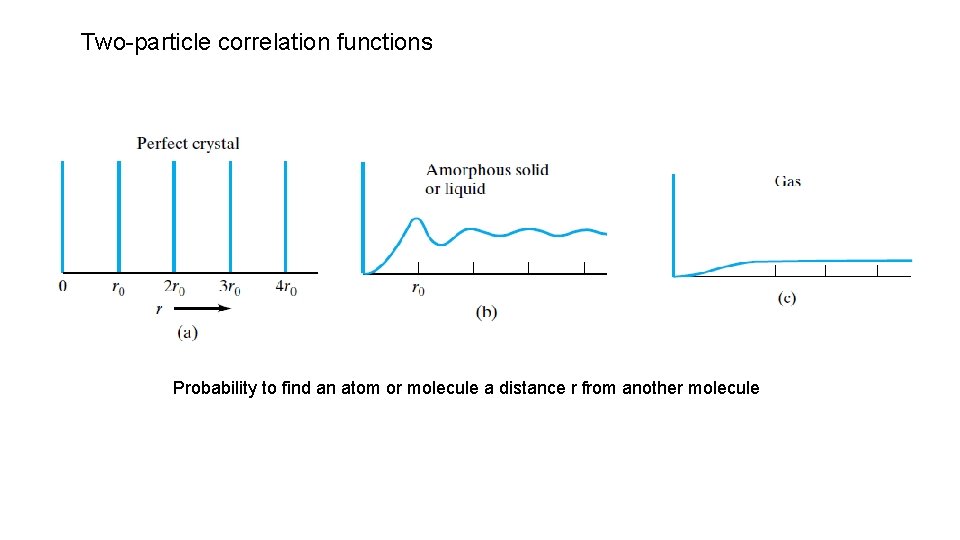
Two-particle correlation functions Probability to find an atom or molecule a distance r from another molecule

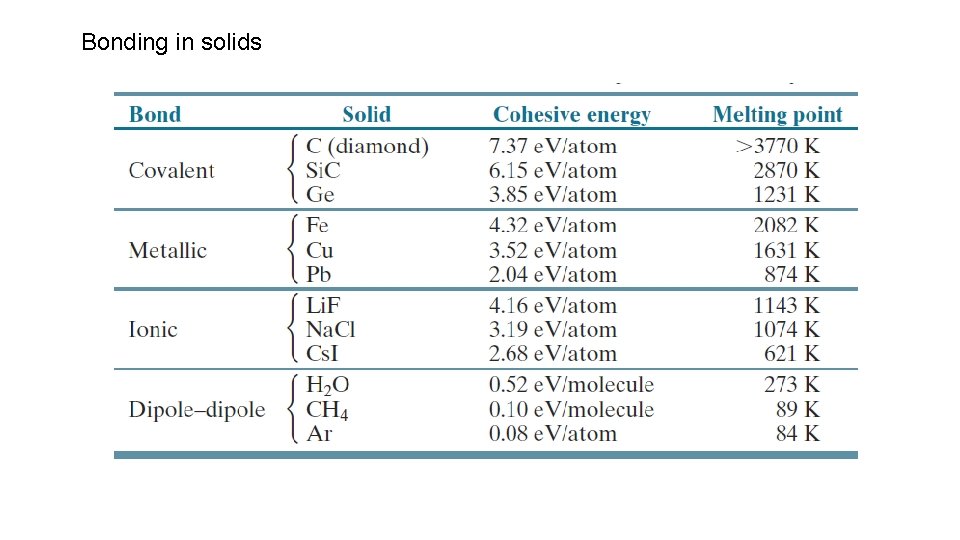
Bonding in solids

Fermi surface of copper kz ky kx

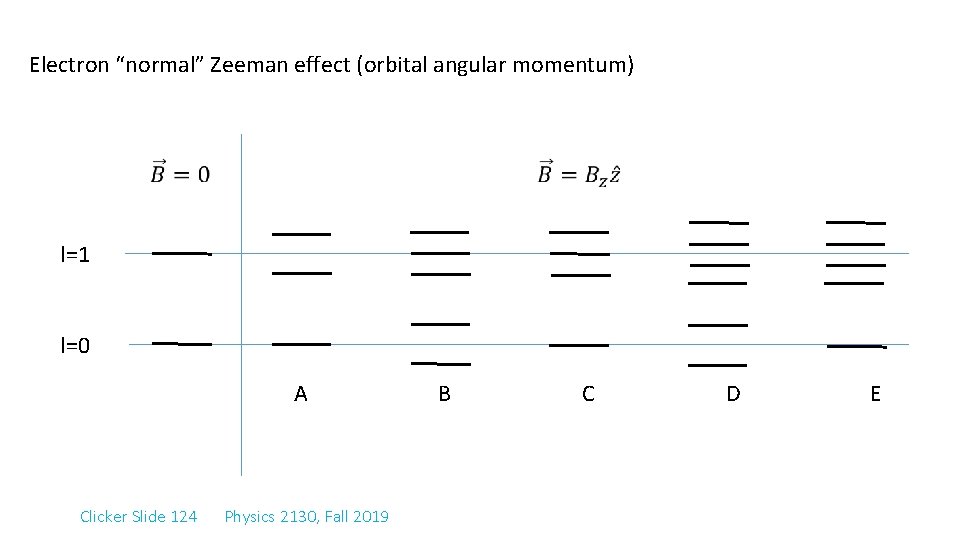
Electron “normal” Zeeman effect (orbital angular momentum) l=1 l=0 A Clicker Slide 124 Physics 2130, Fall 2019 B C D E

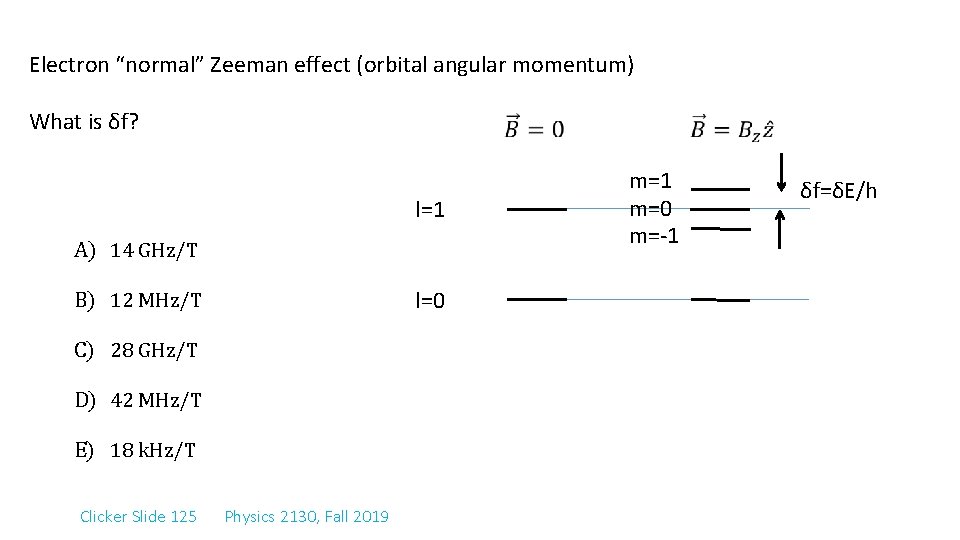
Electron “normal” Zeeman effect (orbital angular momentum) What is δf? l=1 A) 14 GHz/T l=0 B) 12 MHz/T C) 28 GHz/T D) 42 MHz/T E) 18 k. Hz/T Clicker Slide 125 Physics 2130, Fall 2019 m=1 m=0 m=-1 δf=δE/h

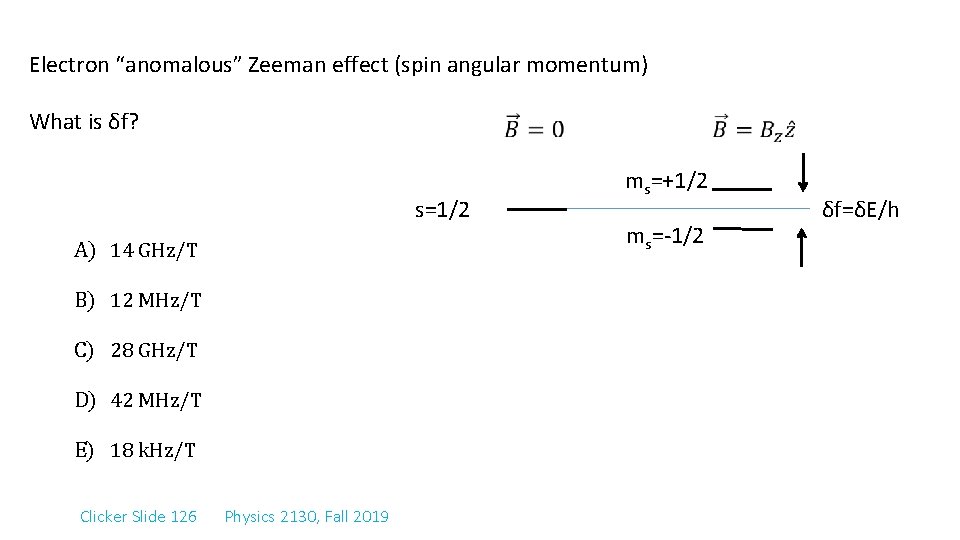
Electron “anomalous” Zeeman effect (spin angular momentum) What is δf? s=1/2 A) 14 GHz/T B) 12 MHz/T C) 28 GHz/T D) 42 MHz/T E) 18 k. Hz/T Clicker Slide 126 Physics 2130, Fall 2019 ms=+1/2 ms=-1/2 δf=δE/h

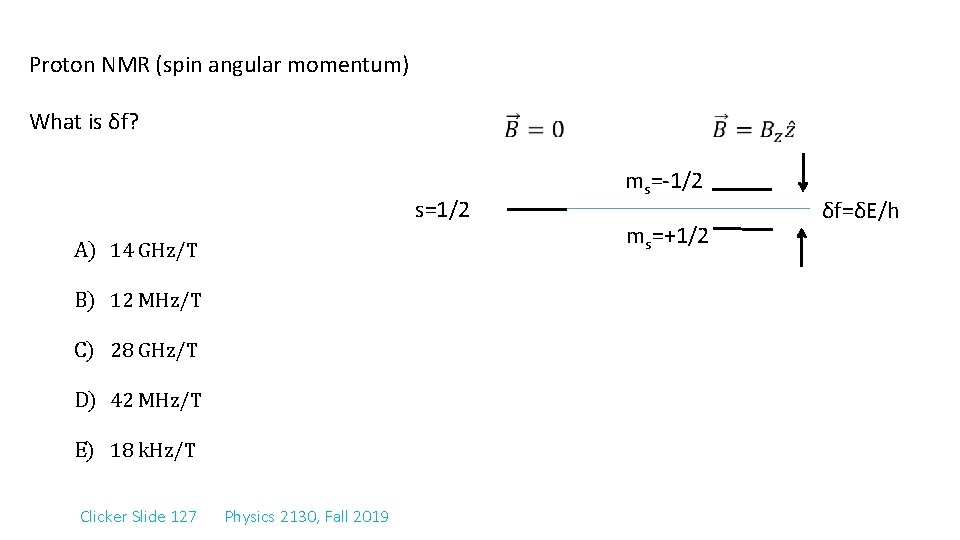
Proton NMR (spin angular momentum) What is δf? s=1/2 A) 14 GHz/T B) 12 MHz/T C) 28 GHz/T D) 42 MHz/T E) 18 k. Hz/T Clicker Slide 127 Physics 2130, Fall 2019 ms=-1/2 ms=+1/2 δf=δE/h

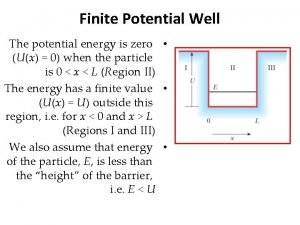
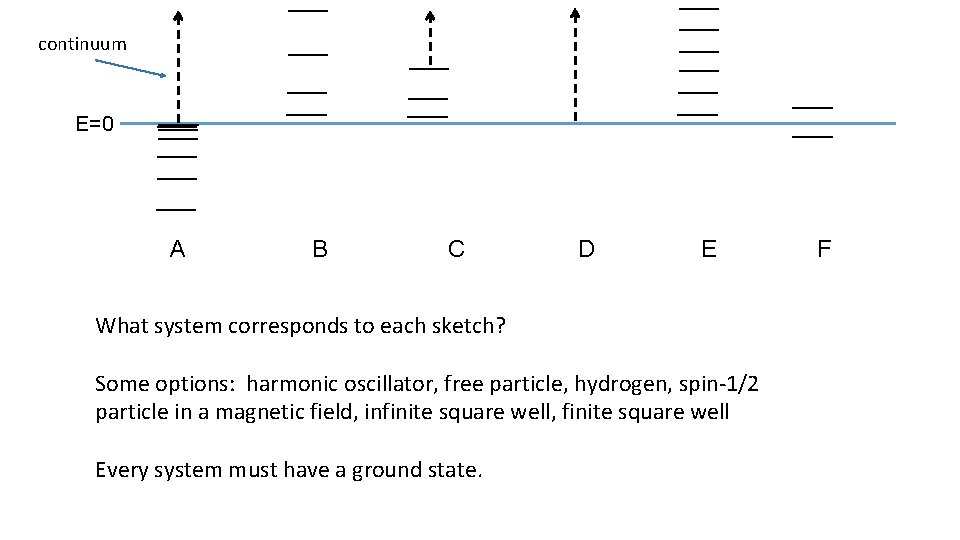
continuum E=0 A B C D E What system corresponds to each sketch? Some options: harmonic oscillator, free particle, hydrogen, spin-1/2 particle in a magnetic field, infinite square well, finite square well Every system must have a ground state. F

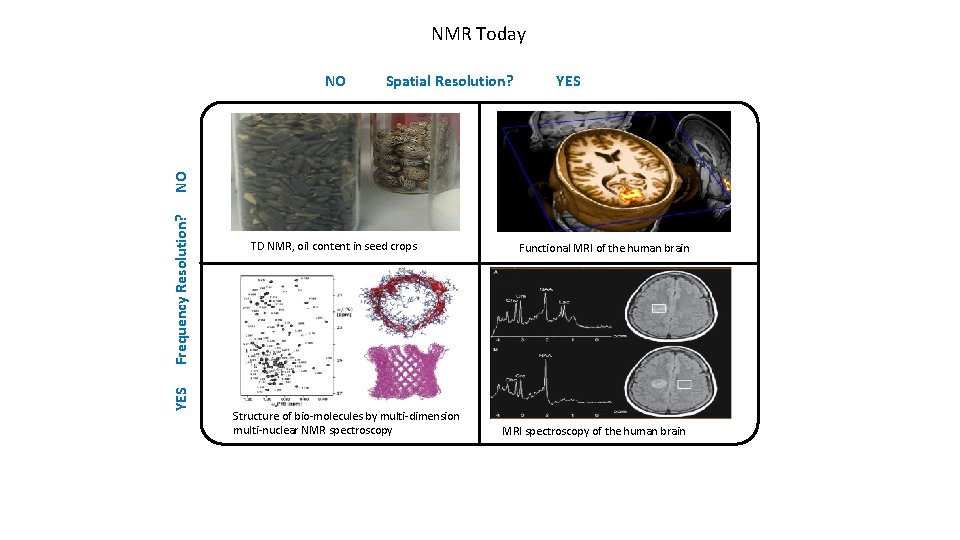
NMR Today Spatial Resolution? YES Frequency Resolution? NO NO TD NMR, oil content in seed crops Structure of bio-molecules by multi-dimension multi-nuclear NMR spectroscopy Functional MRI of the human brain MRI spectroscopy of the human brain

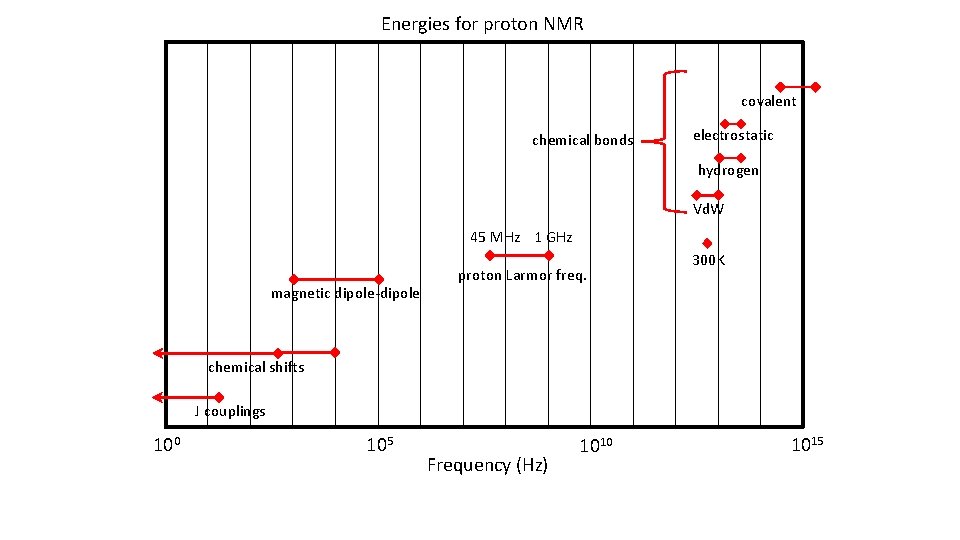
Energies for proton NMR covalent chemical bonds electrostatic hydrogen Vd. W 45 MHz 1 GHz magnetic dipole-dipole proton Larmor freq. 300 K chemical shifts J couplings 100 105 Frequency (Hz) 1010 1015

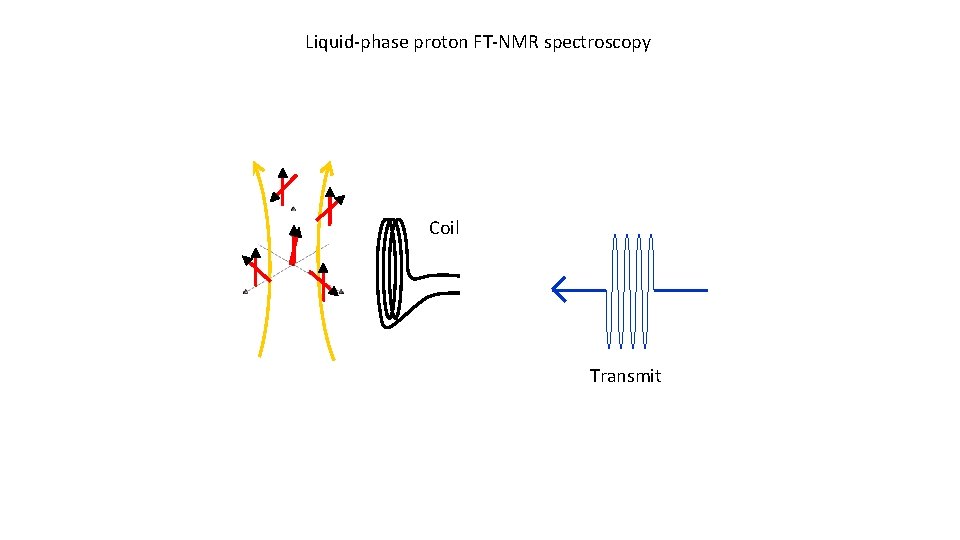
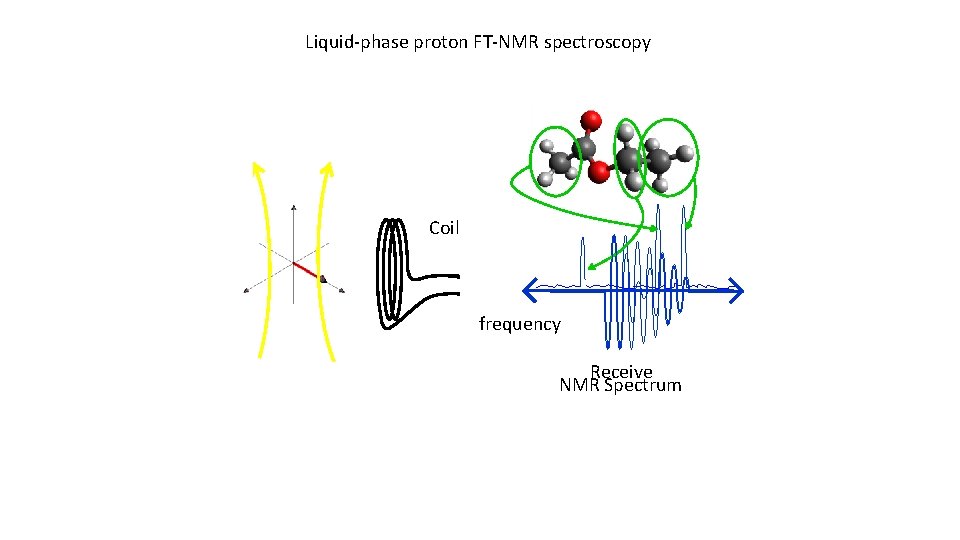
Liquid-phase proton FT-NMR spectroscopy Coil Transmit

Liquid-phase proton FT-NMR spectroscopy Coil frequency Receive NMR Spectrum

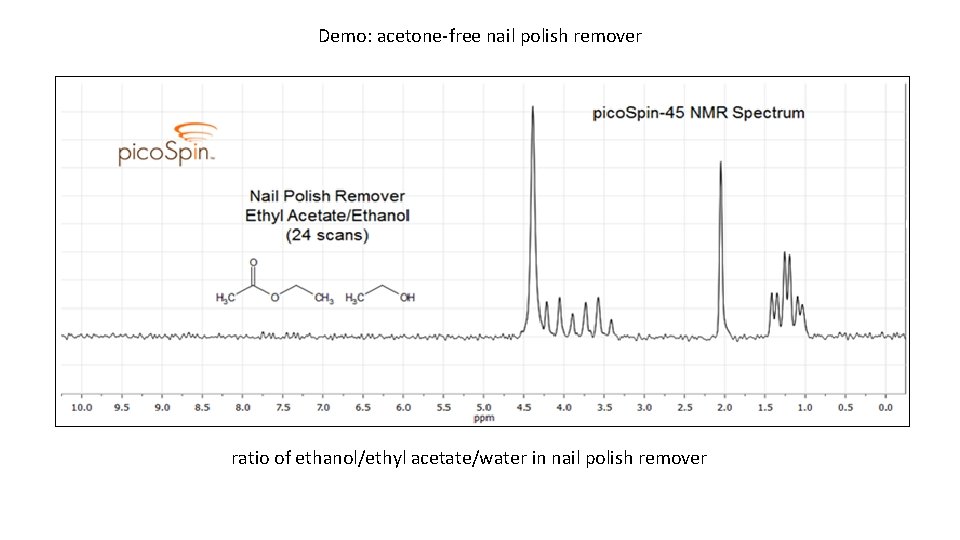
Demo: acetone-free nail polish remover ratio of ethanol/ethyl acetate/water in nail polish remover

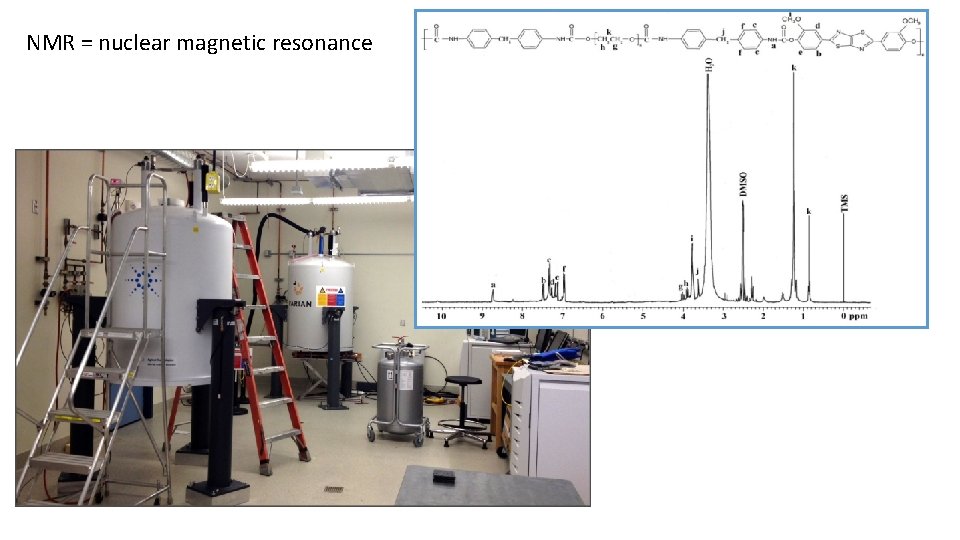
NMR = nuclear magnetic resonance

NMR Spectrometers Today Bruker 400 MHz AVANCE III Varian 900 MHz (¼ Scale) Anasazi Aii 60 MHz Qualion 60 MHz Refinery Analyzer

New Table-top NMR Spectrometers (approx. to scale) 80 MHz (2013) 45 MHz (2010) 8” pico. Spin/Thermo Fisher Oxford 60 MHz (2013) Nanalysis 60 MHz (2012) Magritek 42 MHz (2012) Bruker 60 MHz (2013)

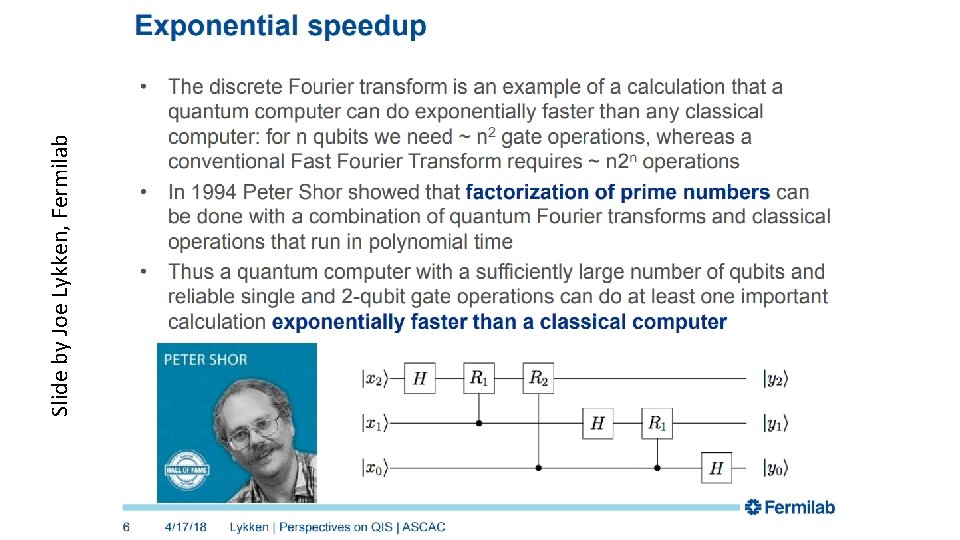
Slide by Joe Lykken, Fermilab

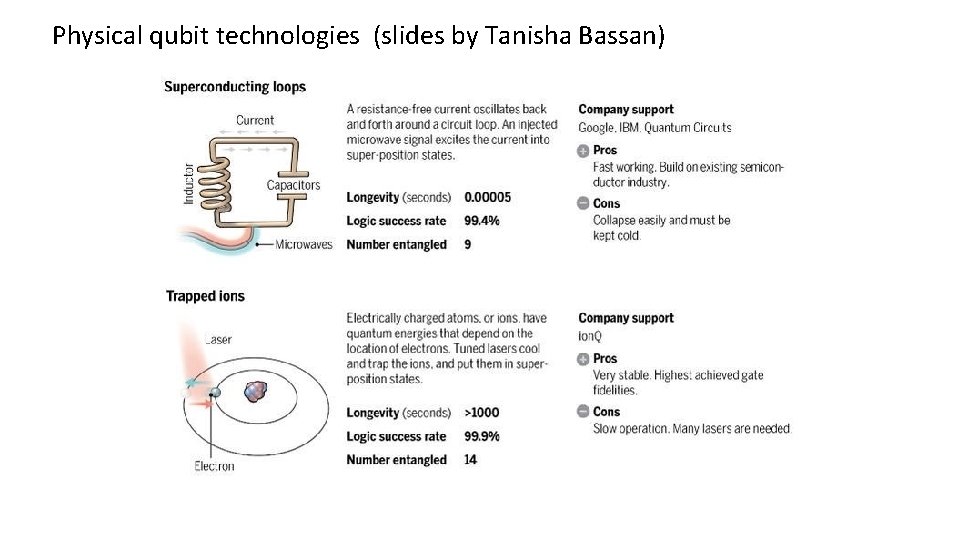
Physical qubit technologies (slides by Tanisha Bassan)

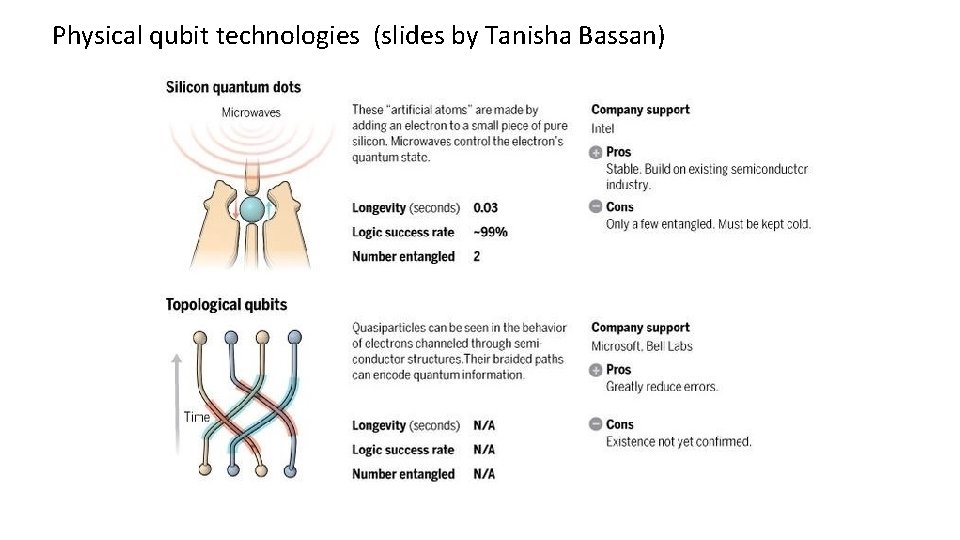
Physical qubit technologies (slides by Tanisha Bassan)

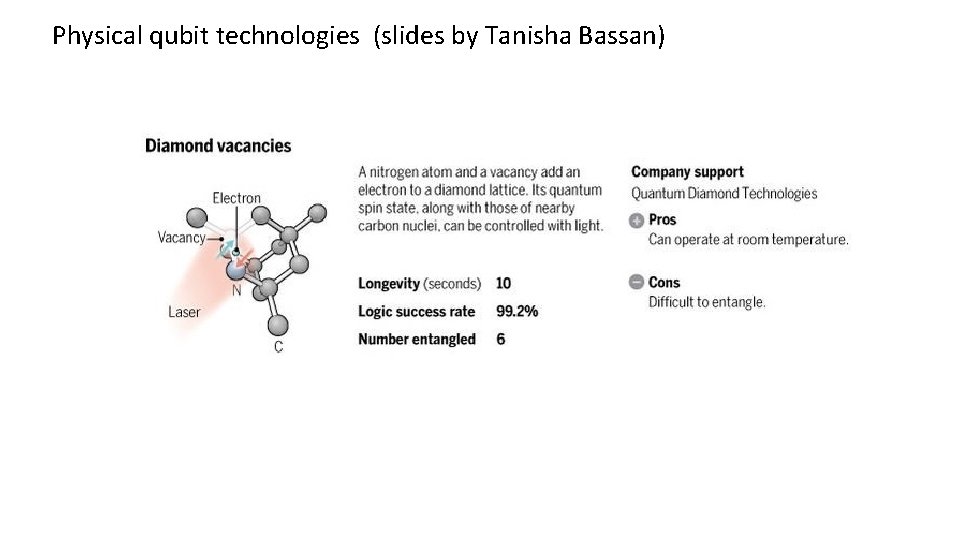
Physical qubit technologies (slides by Tanisha Bassan)

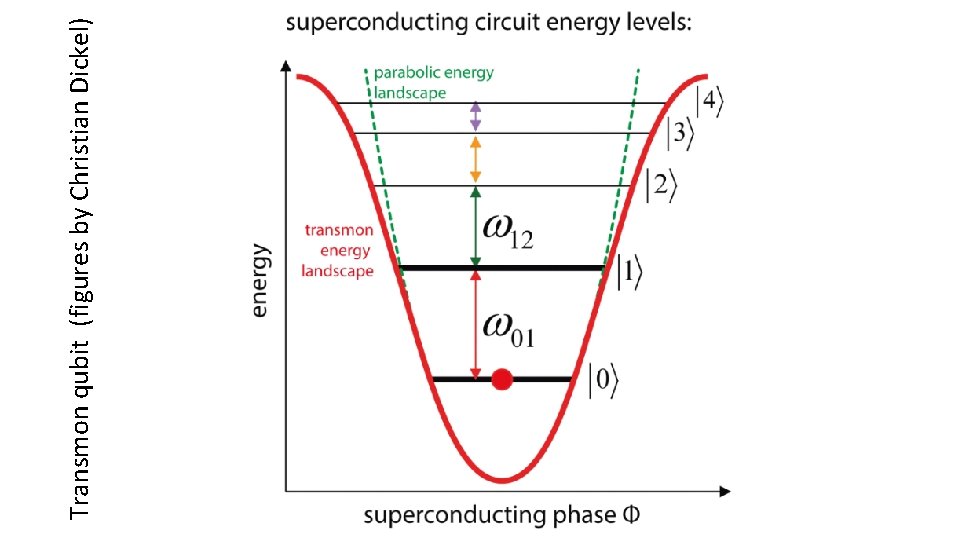
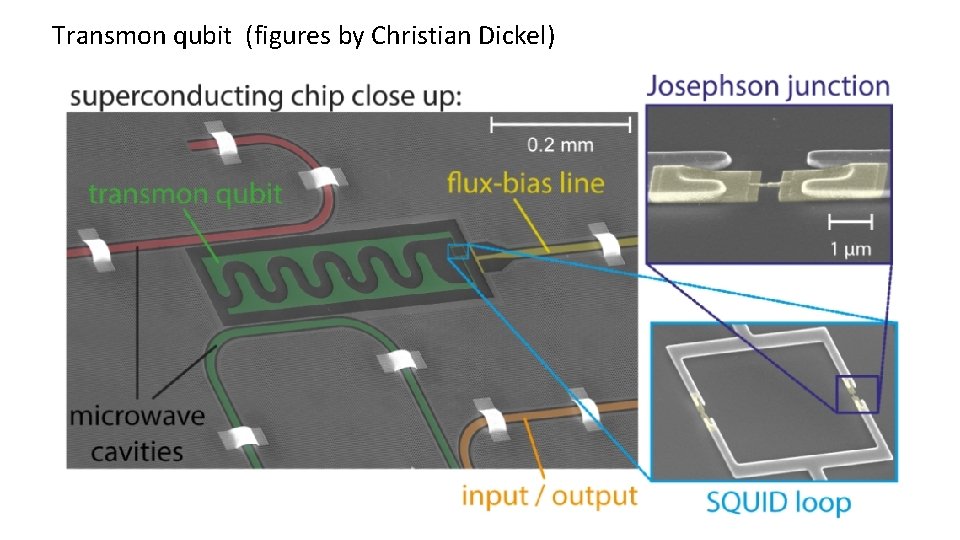
Transmon qubit (figures by Christian Dickel) “transmission line shunted plasma oscillation qubit”

Transmon qubit (figures by Christian Dickel)

Transmon qubit (figures by Christian Dickel)

Amit Vainsencher with Google’s 72 -qubit quantum processor
 Classical mechanics
Classical mechanics Quantum physics vs quantum mechanics
Quantum physics vs quantum mechanics Beta plus decay
Beta plus decay Comp 2130
Comp 2130 Cs 2130
Cs 2130 Modern physics vs classical physics
Modern physics vs classical physics University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Borns interpretation of wave function
Borns interpretation of wave function Expectation value of energy in quantum mechanics
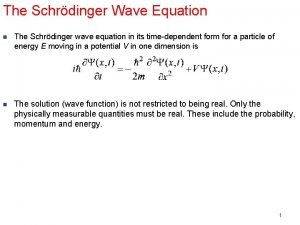
Expectation value of energy in quantum mechanics Incident wave equation
Incident wave equation Expectation value in quantum mechanics
Expectation value in quantum mechanics French and taylor quantum mechanics
French and taylor quantum mechanics Quantum mechanics in your face
Quantum mechanics in your face Operators in quantum chemistry
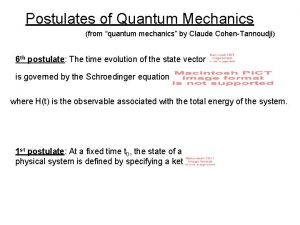
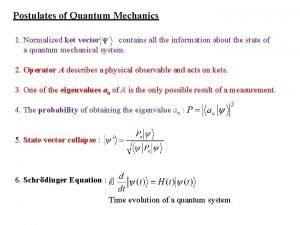
Operators in quantum chemistry Postulates of quantum mechanics
Postulates of quantum mechanics Normalize vector
Normalize vector Operators in quantum mechanics
Operators in quantum mechanics Susan cartwright sheffield
Susan cartwright sheffield Operator formalism in quantum mechanics
Operator formalism in quantum mechanics Schroendiger
Schroendiger Instantons in quantum mechanics
Instantons in quantum mechanics Expectation value in quantum mechanics
Expectation value in quantum mechanics Operators in quantum mechanics
Operators in quantum mechanics Quantum mechanics in three dimensions
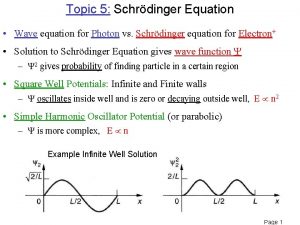
Quantum mechanics in three dimensions The basics of quantum mechanics
The basics of quantum mechanics Grandfather paradox
Grandfather paradox Spin angular momentum formula
Spin angular momentum formula Introduction to quantum statistical mechanics
Introduction to quantum statistical mechanics What is the prison program quantum mechanics
What is the prison program quantum mechanics Commutation relation in quantum mechanics
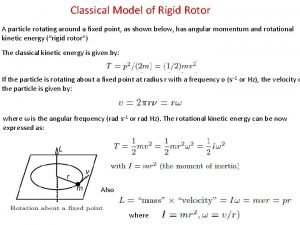
Commutation relation in quantum mechanics 2d rigid rotor
2d rigid rotor Normalize wave function e^ix
Normalize wave function e^ix Commutation relation in quantum mechanics
Commutation relation in quantum mechanics Transfer matrix quantum mechanics
Transfer matrix quantum mechanics Littlejohn quantum mechanics
Littlejohn quantum mechanics Quantum mechanics powerpoint
Quantum mechanics powerpoint Central potential in quantum mechanics
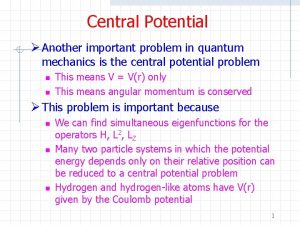
Central potential in quantum mechanics Quantum mechanics
Quantum mechanics Quantum mechanics definition
Quantum mechanics definition Postulates of quantum mechanics
Postulates of quantum mechanics Operator in quantum mechanics
Operator in quantum mechanics Kasap
Kasap Copenhagen interpretation
Copenhagen interpretation