Physics 1161 PreLecture 24 Xrays Section 31 7





- Slides: 15

Physics 1161: Pre-Lecture 24 X-rays • Section 31 -7

X-Rays Photons with energy in approx range 100 e. V to 100, 000 e. V. This large energy means they go right through you (except for your bones). What are the wavelengths? . 01 nm to 10 nm

X-Ray Production How do you produce 100 e. V photons? • Black Body Radiation – Would require temperature over 10 times hotter than surface of sun • Excitation of outer electrons – Typically have energy around 10 e. V • Radioactive Decays – Hard to turn on/off

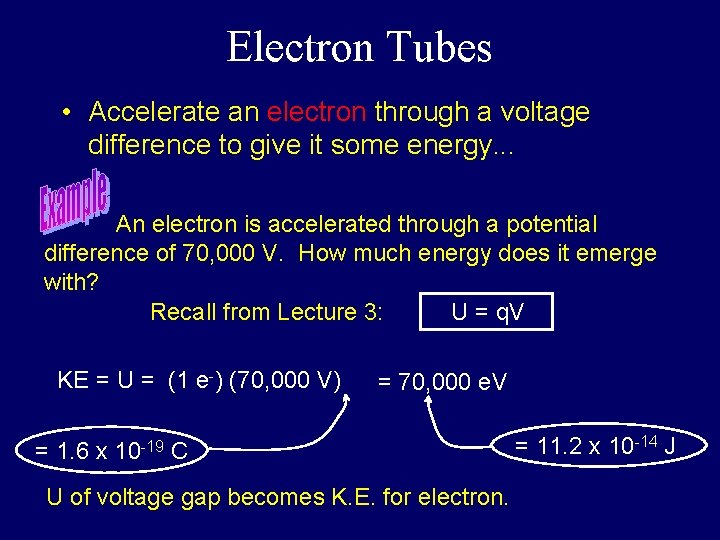
Electron Tubes • Accelerate an electron through a voltage difference to give it some energy. . . An electron is accelerated through a potential difference of 70, 000 V. How much energy does it emerge with? Recall from Lecture 3: U = q. V KE = U = (1 e-) (70, 000 V) = 70, 000 e. V = 1. 6 x 10 -19 C U of voltage gap becomes K. E. for electron. = 11. 2 x 10 -14 J

From Electrons to X-Rays • Now take these high energy electrons (up to 100, 000 e. V) and slam them into heavy atoms - any element. • 2 kinds of X-Rays are produced: – “Bremsstrahlung” – “Characteristic”

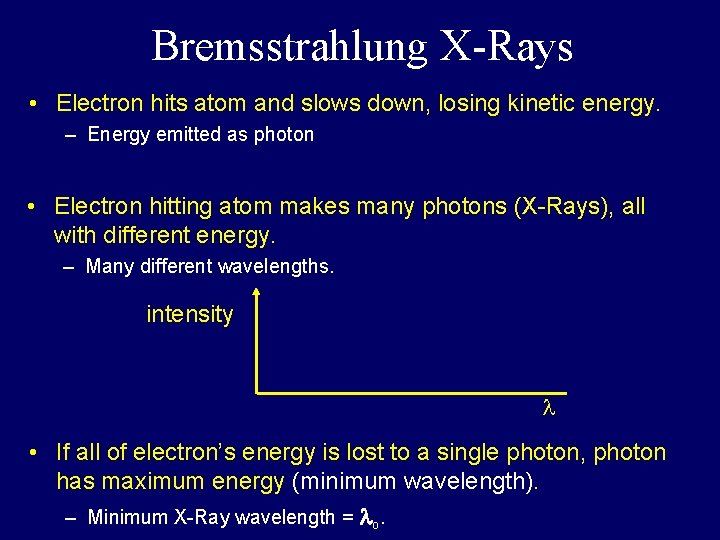
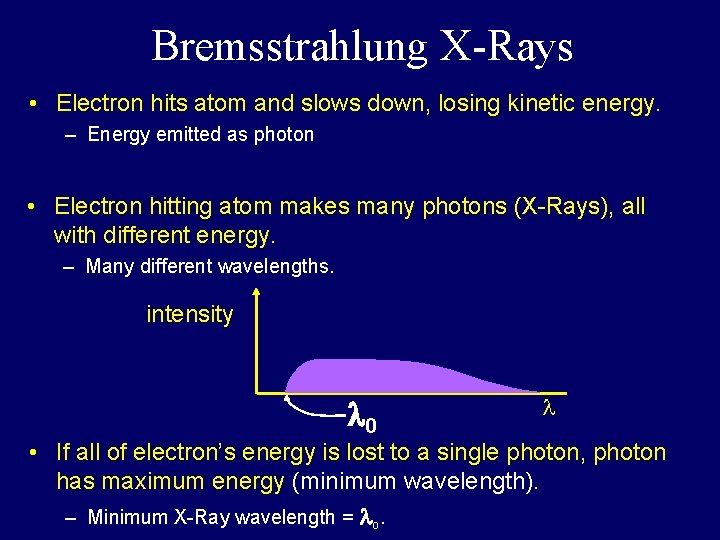
Bremsstrahlung X-Rays • Electron hits atom and slows down, losing kinetic energy. – Energy emitted as photon • Electron hitting atom makes many photons (X-Rays), all with different energy. – Many different wavelengths. intensity • If all of electron’s energy is lost to a single photon, photon has maximum energy (minimum wavelength). – Minimum X-Ray wavelength = o.

Bremsstrahlung X-Rays • Electron hits atom and slows down, losing kinetic energy. – Energy emitted as photon • Electron hitting atom makes many photons (X-Rays), all with different energy. – Many different wavelengths. intensity 0 • If all of electron’s energy is lost to a single photon, photon has maximum energy (minimum wavelength). – Minimum X-Ray wavelength = o.

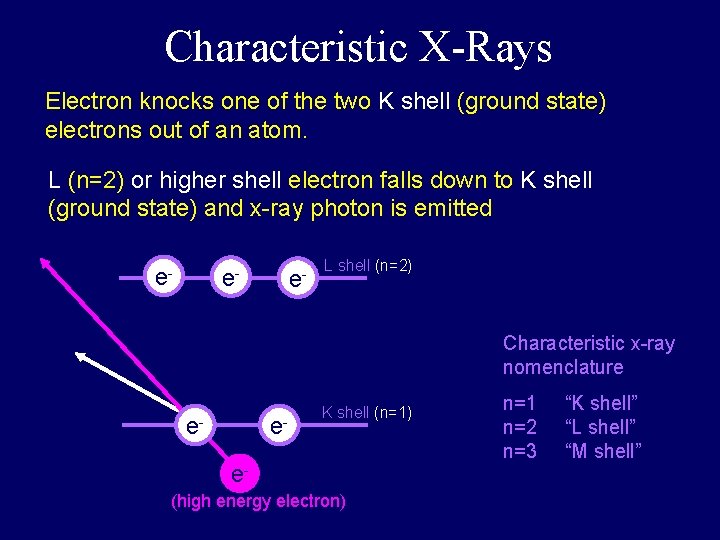
Characteristic X-Rays Electron knocks one of the two K shell (ground state) electrons out of an atom. L (n=2) or higher shell electron falls down to K shell (ground state) and x-ray photon is emitted e- e- e- L shell (n=2) Characteristic x-ray nomenclature e- e- K shell (n=1) e(high energy electron) n=1 n=2 n=3 “K shell” “L shell” “M shell”

Characteristic X-Rays Electron knocks one of the two K shell (ground state) electrons out of an atom. L (n=2) or higher shell electron falls down to K shell (ground state) and x-ray photon is emitted e- e- e- L shell (n=2) eejected electron Characteristic x-ray nomenclature ee- e- K shell (n=1) n=1 n=2 n=3 “K shell” “L shell” “M shell”

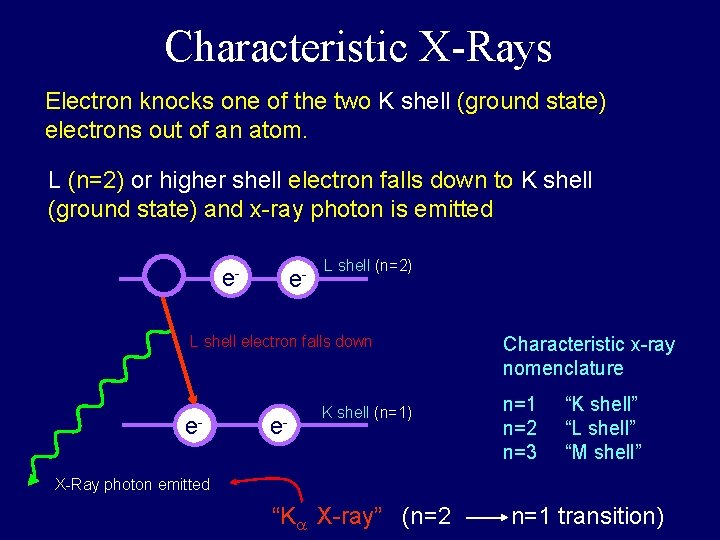
Characteristic X-Rays Electron knocks one of the two K shell (ground state) electrons out of an atom. L (n=2) or higher shell electron falls down to K shell (ground state) and x-ray photon is emitted e- e- e- L shell (n=2) L shell electron falls down e- e- K shell (n=1) Characteristic x-ray nomenclature n=1 n=2 n=3 “K shell” “L shell” “M shell” X-Ray photon emitted “K X-ray” (n=2 n=1 transition)

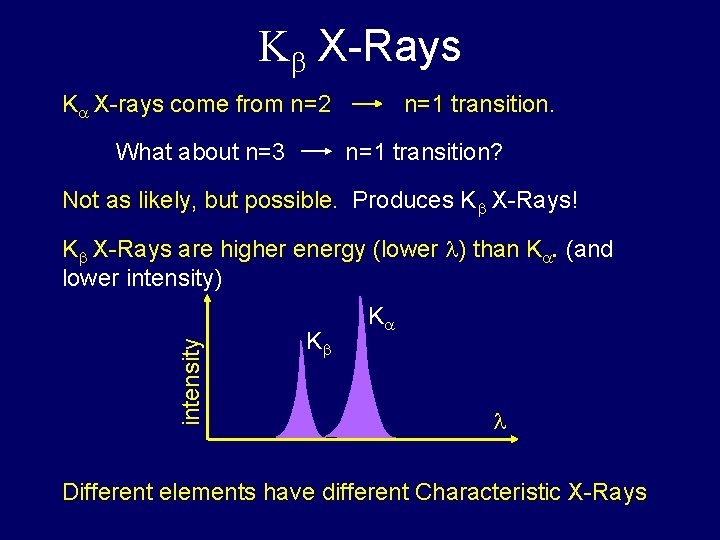
Kb X-Rays K X-rays come from n=2 What about n=3 n=1 transition? Not as likely, but possible. Produces Kb X-Rays! intensity Kb X-Rays are higher energy (lower ) than K. (and lower intensity) Kb K Different elements have different Characteristic X-Rays

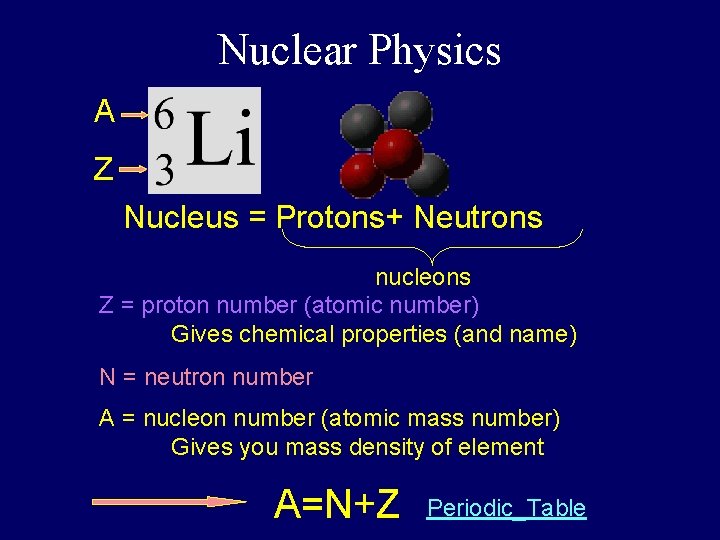
Nuclear Physics A Z Nucleus = Protons+ Neutrons nucleons Z = proton number (atomic number) Gives chemical properties (and name) N = neutron number A = nucleon number (atomic mass number) Gives you mass density of element A=N+Z Periodic_Table

Strong Nuclear Force • Acts on Protons and Neutrons • Strong enough to overcome Coulomb repulsion • Acts over very short distances Two atoms don’t feel force

Strong Nuclear Force Hydrogen atom: Binding energy =13. 6 e. V (of electron to nucleus) Coulomb force proton electron proton neutron Simplest Nucleus: Deuteron=neutron+proton Very strong force Binding energy of deuteron = 2. 2 Mev! That’s around 200, 000 times bigger! or

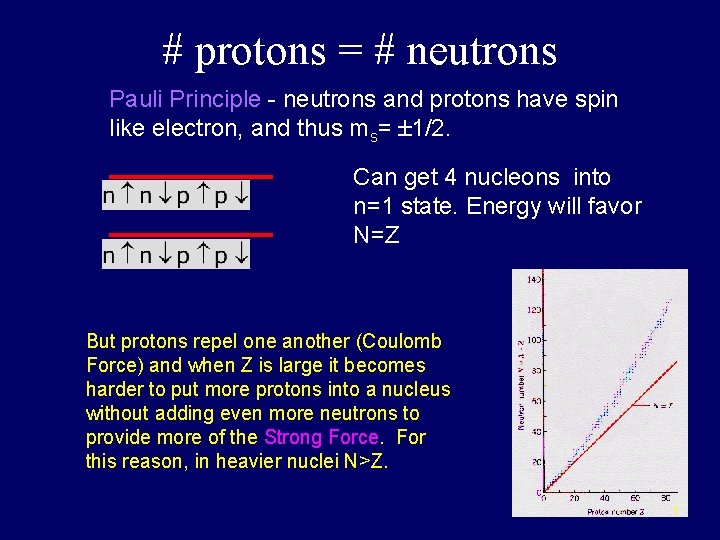
# protons = # neutrons Pauli Principle - neutrons and protons have spin like electron, and thus ms= 1/2. Can get 4 nucleons into n=1 state. Energy will favor N=Z But protons repel one another (Coulomb Force) and when Z is large it becomes harder to put more protons into a nucleus without adding even more neutrons to provide more of the Strong Force. For this reason, in heavier nuclei N>Z. 7