PHYSICS 111 THERMODYNAMICS Review Problem 1 Find the

PHYSICS 111 – THERMODYNAMICS

Review Problem 1: Find the work done if the force is 45. 0 -N, the angle is 50. 0 degrees, and the displacement is 75. 0

Solution: W = F cos degree * S = 45 cos 50 * 75 = 2170 Joules

Review Problem 2: Starting from rest, two skaters push off against each other on ice where friction is negligible. One is a 54 -kg woman and one is a 88 kg man. The woman moves away with a speed of +2. 5 m/s. Find the recoil velocity of the man.

Solution: P=p Mv =mv V = - mv /m V= - 54*25 / 88 = -1. 5 m/s

Final Review Problem: A 0. 25 -kg ball attached to a string is rotating in a horizontal circle of radius 0. 5 m. If the ball revolves twice every second, what is the tension in the

Solution: F tension = F centripetal force = ma = m v^2/ r V= 2 pie / T = 2 pie / 5 = 6. 28 m/s Ft=. 25 ( 6. 28 ^2 /. 5) = 20 N
Fill in the Chart Unit: Isobaric Isochoric Isothermal Adiabatic What is Constant? Value of work, Value of Heat W exchange, Q Value of Internal Energy Change, U
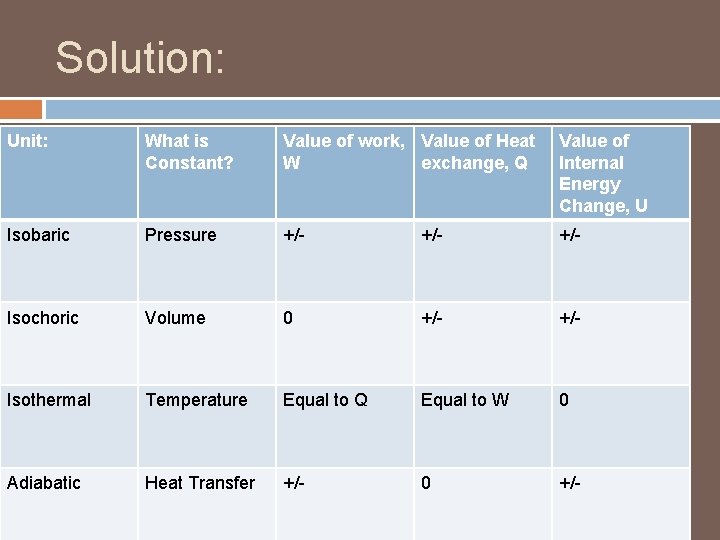
Solution: Unit: What is Constant? Value of work, Value of Heat W exchange, Q Value of Internal Energy Change, U Isobaric Pressure +/- +/- Isochoric Volume 0 +/- Isothermal Temperature Equal to Q Equal to W 0 Adiabatic Heat Transfer +/- 0 +/-

Today ‘s Material An ideal gas absorbs 238 J of heat as it performs 845 J of work. What is the resulting change in temperature if there are 2. 4 moles of an ideal gas in the system?

Solution: Q= +238 W=+845 U = 238 – 845 =-607 Joules U = 3/2 n. RT T = 2/3 U / n. R = 2/3 ( -607) / ( 2. 4 * 8. 31) T = -20 Kelvin

Practice Problem 2: A system containing an ideal gas at a constant pressure of 2. 2 × 10^5 Pa gains 2431 J of heat. During the process, the internal energy of the system increases by 1243 J. What is the change in volume of the

Solution: P = 2. 2 × 10^5 Pa Q = + 2431 J U = + 1243 J W = Q – U = 2431 -1243 =1188 W = PV V= W/P = 1188/ 2. 2 * 10 ^ 5 = 5. 4 *10^ -3

LAST PROBLEM!!!! What is the specific heat of a metal if its mass is 26. 86 g and it requires 418. 6 J of heat energy to raise its temperature from 27. 4 to 67. 3 degrees Celsius.

SOLUTION: 418. 6 J = 26. 86 (C) (67. 3 – 27. 4) C =. 391 J/ gram Celsius
- Slides: 15