Physics 102 Lecture 25 Periodic Table Atomic Structure
Physics 102: Lecture 25 Periodic Table, Atomic Structure Physics 102: Lecture 25, Slide 1
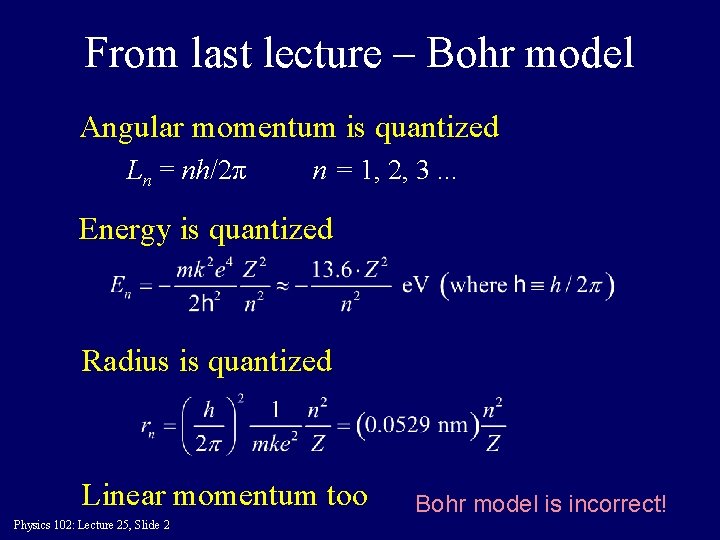
From last lecture – Bohr model Angular momentum is quantized Ln = nh/2π n = 1, 2, 3. . . Energy is quantized Radius is quantized Linear momentum too Physics 102: Lecture 25, Slide 2 Bohr model is incorrect!
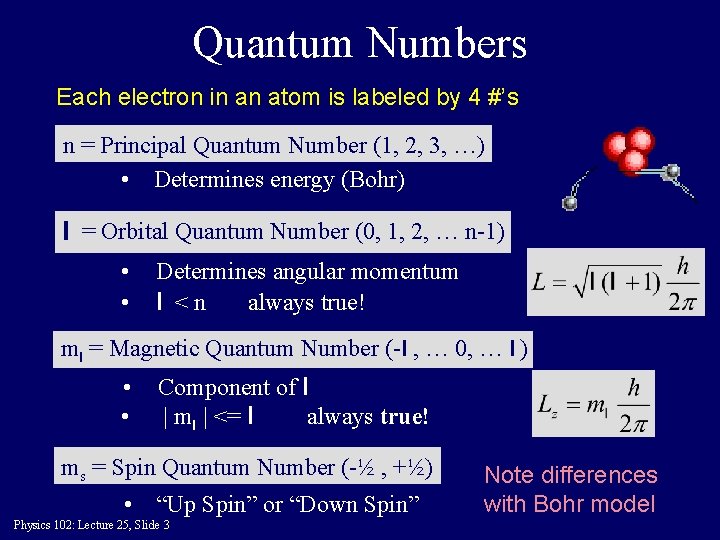
Quantum Numbers Each electron in an atom is labeled by 4 #’s n = Principal Quantum Number (1, 2, 3, …) • Determines energy (Bohr) l = Orbital Quantum Number (0, 1, 2, … n-1) • • Determines angular momentum l <n always true! ml = Magnetic Quantum Number (-l , … 0, … l ) • • Component of l | ml | <= l always true! ms = Spin Quantum Number (-½ , +½) • “Up Spin” or “Down Spin” Physics 102: Lecture 25, Slide 3 Note differences with Bohr model

ACT: Quantum numbers For which state of hydrogen is the orbital angular momentum required to be zero? 1. n=1 2. n=2 3. n=3 Physics 102: Lecture 25, Slide 4

Spectroscopic Nomenclature “Shells” “Subshells” l =0 is “s state” l =1 is “p state” l =2 is “d state” l =3 is “f state” l =4 is “g state” n=1 is “K shell” n=2 is “L shell” n=3 is “M shell” n=4 is “N shell” n=5 is “O shell” 1 electron in ground state of Hydrogen: n=1, l =0 is denoted as: 1 s 1 n=1 Physics 102: Lecture 25, Slide 5 l =0 1 electron
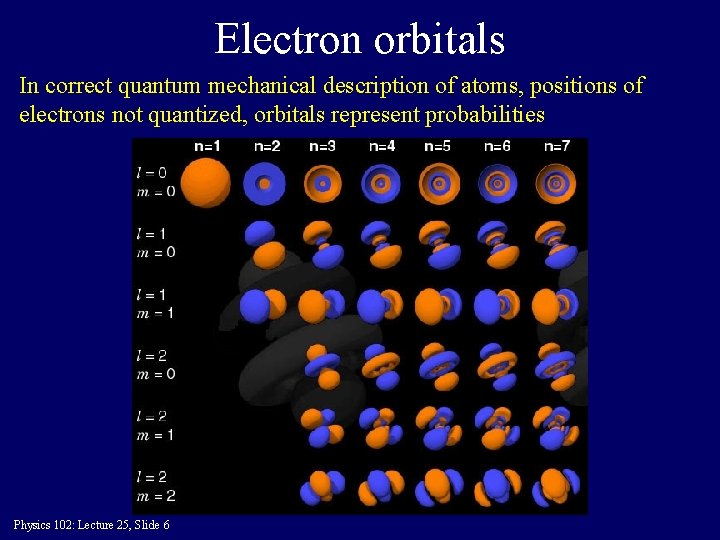
Electron orbitals In correct quantum mechanical description of atoms, positions of electrons not quantized, orbitals represent probabilities Physics 102: Lecture 25, Slide 6
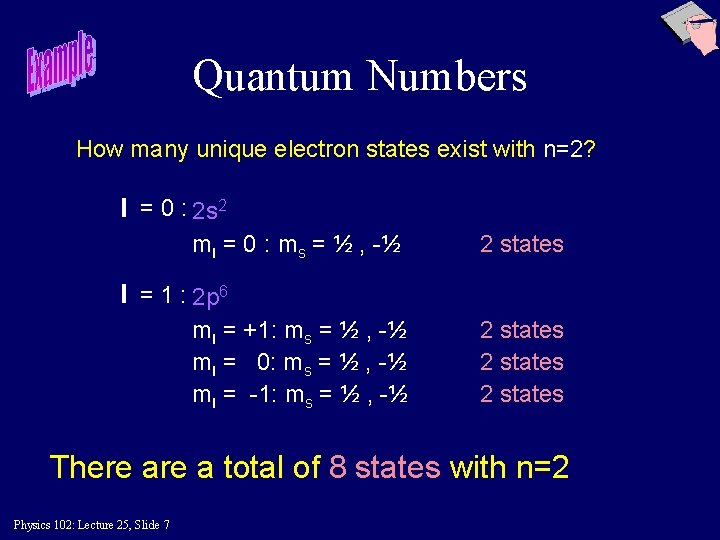
Quantum Numbers How many unique electron states exist with n=2? l = 0 : 2 s 2 ml = 0 : ms = ½ , -½ 2 states l = 1 : 2 p 6 ml = +1: ms = ½ , -½ ml = 0: ms = ½ , -½ ml = -1: ms = ½ , -½ 2 states There a total of 8 states with n=2 Physics 102: Lecture 25, Slide 7

ACT: Quantum Numbers How many unique electron states exist with n=5 and ml = +3? A) 0 B) 4 C) 8 D) 16 E) 50 Physics 102: Lecture 25, Slide 8

Preflight 25. 2 What is the maximum number of electrons that can exist in the 5 g (n=5, l =4) subshell of an atom? Physics 102: Lecture 25, Slide 9
Pauli Exclusion Principle In an atom with many electrons only one electron is allowed in each quantum state (n, l, ms). This explains the periodic table! Physics 102: Lecture 25, Slide 10
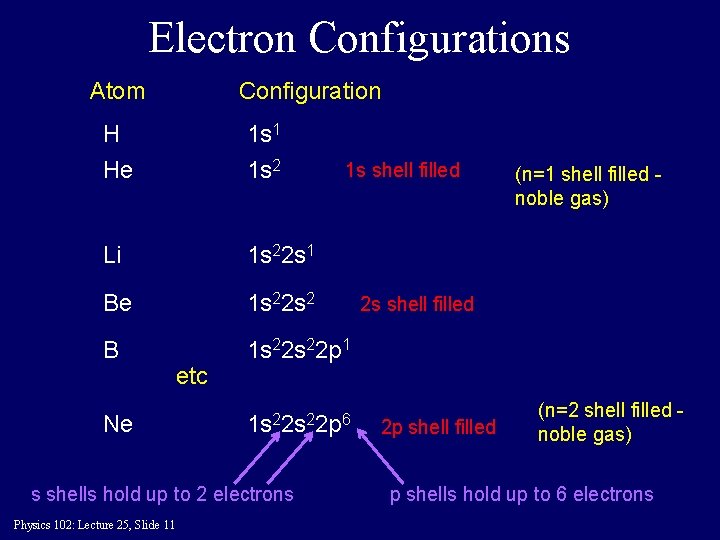
Electron Configurations Atom Configuration H 1 s 1 He 1 s 2 Li 1 s 22 s 1 Be 1 s 22 s 2 B 1 s 22 p 1 1 s shell filled (n=1 shell filled noble gas) 2 s shell filled etc Ne 1 s 22 p 6 s shells hold up to 2 electrons Physics 102: Lecture 25, Slide 11 2 p shell filled (n=2 shell filled noble gas) p shells hold up to 6 electrons
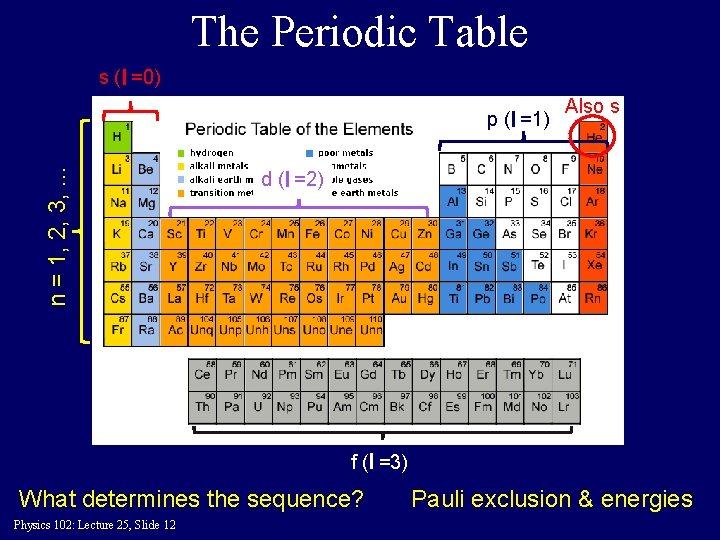
The Periodic Table s (l =0) n = 1, 2, 3, . . . p (l =1) Also s d (l =2) f (l =3) What determines the sequence? Physics 102: Lecture 25, Slide 12 Pauli exclusion & energies
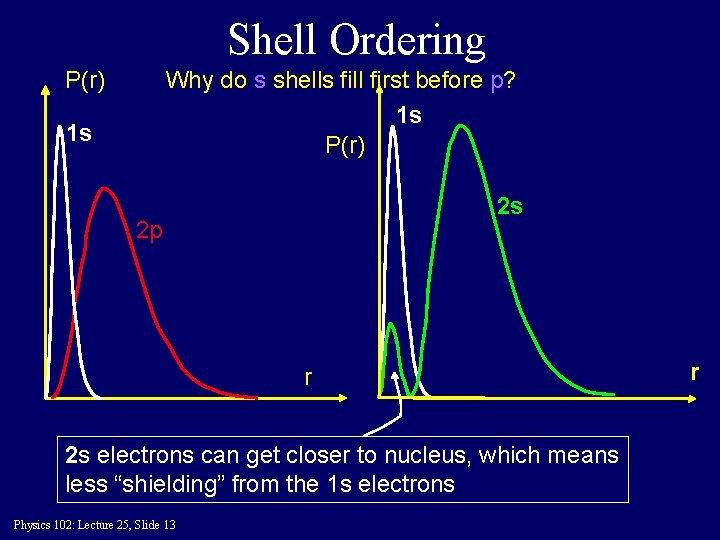
Shell Ordering P(r) Why do s shells fill first before p? 1 s P(r) 1 s 2 s 2 p r 2 s electrons can get closer to nucleus, which means less “shielding” from the 1 s electrons Physics 102: Lecture 25, Slide 13 r
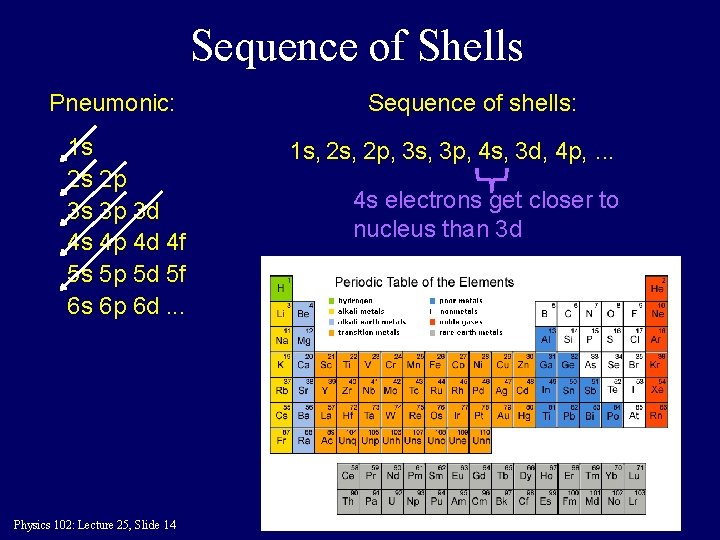
Sequence of Shells Pneumonic: 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d. . . Physics 102: Lecture 25, Slide 14 Sequence of shells: 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, . . . 4 s electrons get closer to nucleus than 3 d
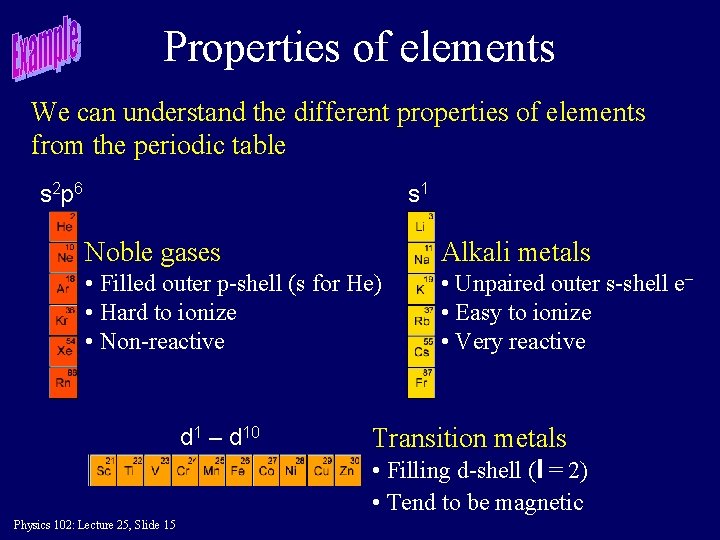
Properties of elements We can understand the different properties of elements from the periodic table s 2 p 6 s 1 Noble gases Alkali metals • Filled outer p-shell (s for He) • Hard to ionize • Non-reactive • Unpaired outer s-shell e– • Easy to ionize • Very reactive d 1 – d 10 Transition metals • Filling d-shell (l = 2) • Tend to be magnetic Physics 102: Lecture 25, Slide 15
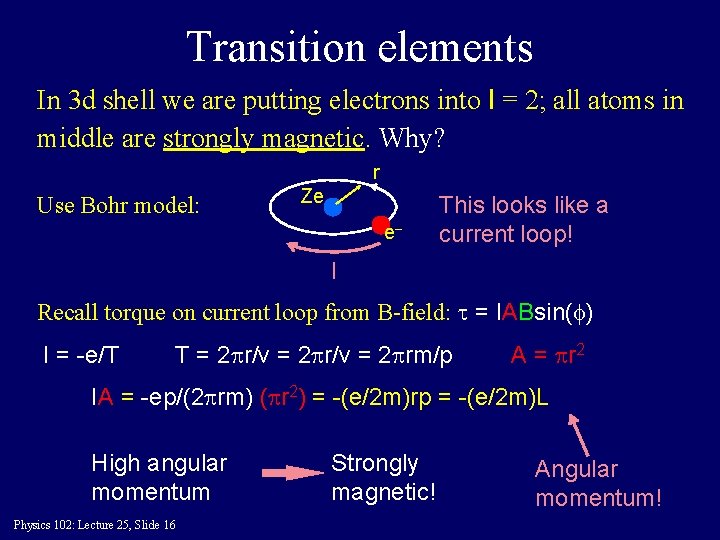
Transition elements In 3 d shell we are putting electrons into l = 2; all atoms in middle are strongly magnetic. Why? r Use Bohr model: Ze e– This looks like a current loop! I Recall torque on current loop from B-field: t = IABsin(f) I = -e/T T = 2 pr/v = 2 prm/p A = pr 2 IA = -ep/(2 prm) (pr 2) = -(e/2 m)rp = -(e/2 m)L High angular momentum Physics 102: Lecture 25, Slide 16 Strongly magnetic! Angular momentum!

Sodium Na 1 s 22 p 6 3 s 1 Single outer electron Neon - like core Many spectral lines of Na are outer electron making transitions Yellow line of Na flame test is 3 p 3 s Physics 102: Lecture 25, Slide 17 www. webelements. com/w ebelements/scholar/index. html
Summary • Each electron state labeled by 4 numbers: n = principal quantum number (1, 2, 3, …) l = angular momentum (0, 1, 2, … n-1) ml = component of l (-l < ml < l) ms = spin (-½ , +½) • Pauli Exclusion Principle explains periodic table • Shells fill in order of lowest energy. Physics 102: Lecture 25, Slide 18
- Slides: 18