Physician Reference Slides 2007 US Filler Market Evolution













- Slides: 56

Physician Reference Slides 2007

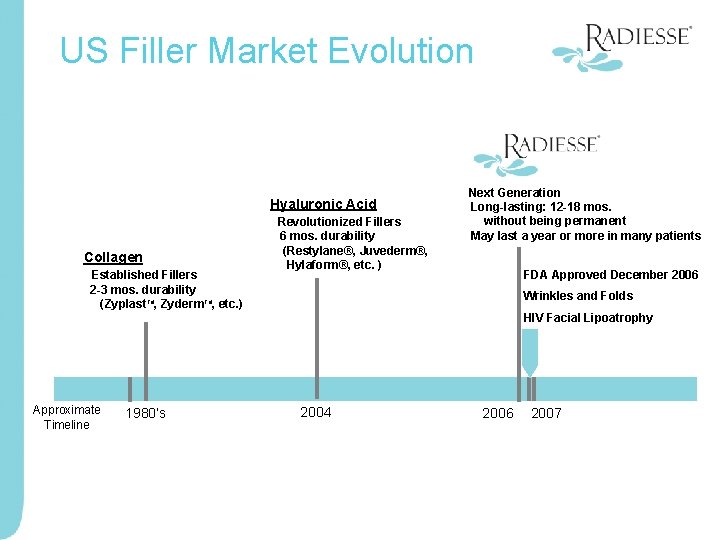
US Filler Market Evolution Hyaluronic Acid Collagen Established Fillers 2 -3 mos. durability (Zyplast. TM, Zyderm. TM, etc. ) Approximate Timeline 1980’s Revolutionized Fillers 6 mos. durability (Restylane®, Juvederm®, Hylaform®, etc. ) Next Generation Long-lasting: 12 -18 mos. without being permanent May last a year or more in many patients FDA Approved December 2006 Wrinkles and Folds HIV Facial Lipoatrophy 2004 2006 2007

US Regulatory Approvals Radiesse received approval from the FDA December 26, 2006 for facial soft tissue augmentation – Treatment of facial wrinkles and folds, such as nasolabial folds, marionette lines, etc. – Correction of facial wasting as a result of HIV-associated Lipoatrophy Radiesse is the first dermal filler to receive FDA approval for two facial aesthetic applications

Radiesse is a safe, next generation cosmetic dermal filler aiming to become the new standard for long lasting correction of facial lines and wrinkles. Clinical studies prove that in many patients Radiesse lasts a year or longer and delivers a natural look that results in very high patient satisfaction. Synthetic Calcium Hydroxylapatite (Ca. HA) microspheres [30%] suspended in a carboxy-methylcellulose resorbable aqueous gel carrier [70%] Stimulates the body to produce new collagen Pre-filled 1. 3 cc & 0. 3 cc syringes No skin or allergy testing No special handling requirements

Radiesse Not Only Fills, but Volumizes When placed into soft tissue, Radiesse provides immediate correction – Gel + Ca. HA Particles Over time the gel is resorbed and the Ca. HA particles stimulate the body to produce collagen – Collagen + Ca. HA Particles Decalcified 25µm to 45 µm particles

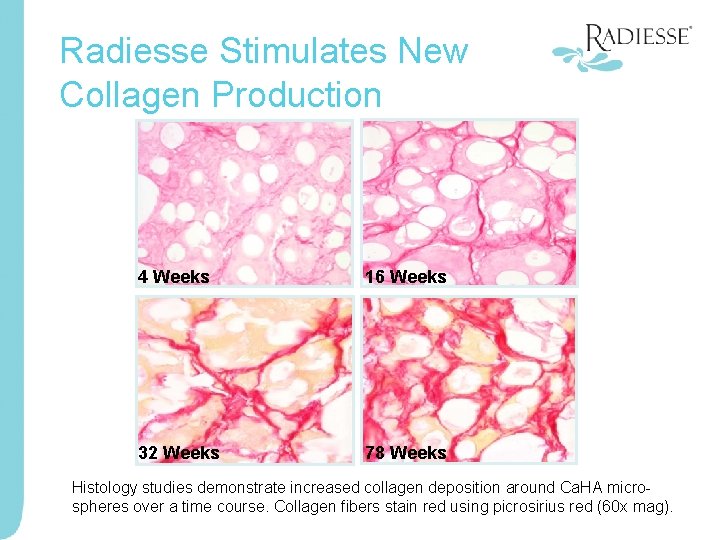
Radiesse Stimulates New Collagen Production 4 Weeks 16 Weeks 32 Weeks 78 Weeks Histology studies demonstrate increased collagen deposition around Ca. HA microspheres over a time course. Collagen fibers stain red using picrosirius red (60 x mag).

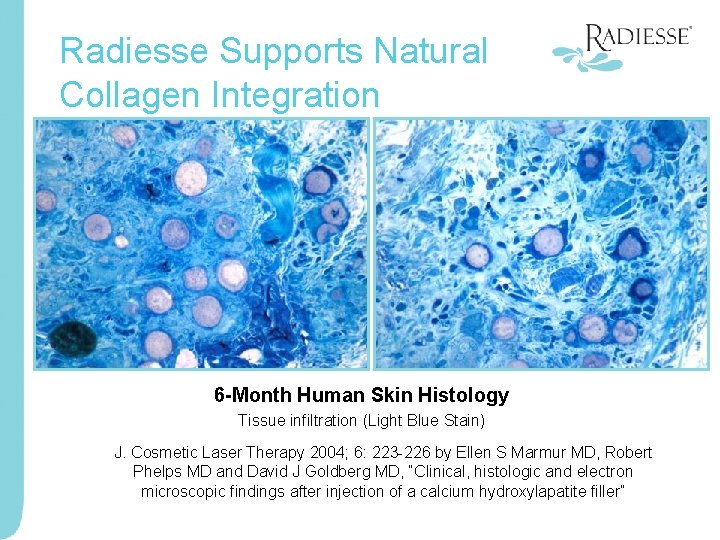
Radiesse Supports Natural Collagen Integration 6 -Month Human Skin Histology Tissue infiltration (Light Blue Stain) J. Cosmetic Laser Therapy 2004; 6: 223 -226 by Ellen S Marmur MD, Robert Phelps MD and David J Goldberg MD, “Clinical, histologic and electron microscopic findings after injection of a calcium hydroxylapatite filler”

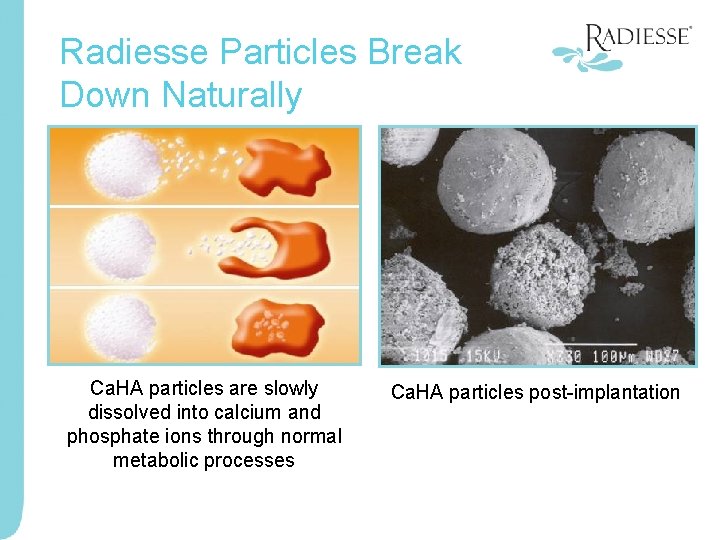
Radiesse Particles Break Down Naturally Ca. HA particles are slowly dissolved into calcium and phosphate ions through normal metabolic processes Ca. HA particles post-implantation

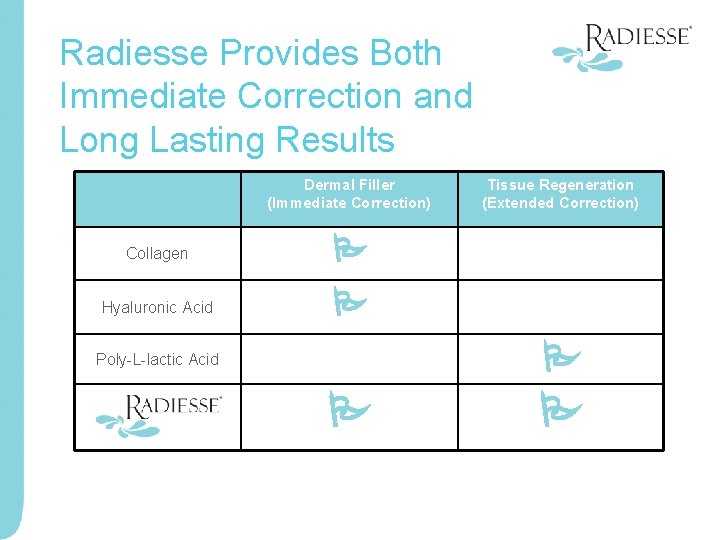
Radiesse Provides Both Immediate Correction and Long Lasting Results Dermal Filler (Immediate Correction) Collagen Hyaluronic Acid Tissue Regeneration (Extended Correction) Poly-L-lactic Acid

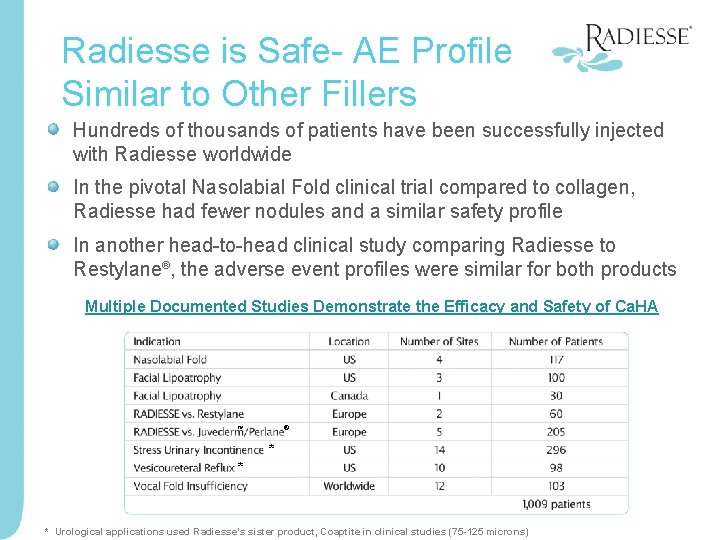
Radiesse is Safe- AE Profile Similar to Other Fillers Hundreds of thousands of patients have been successfully injected with Radiesse worldwide In the pivotal Nasolabial Fold clinical trial compared to collagen, Radiesse had fewer nodules and a similar safety profile In another head-to-head clinical study comparing Radiesse to Restylane®, the adverse event profiles were similar for both products Multiple Documented Studies Demonstrate the Efficacy and Safety of Ca. HA ® ™ * * * Urological applications used Radiesse’s sister product, Coaptite in clinical studies (75 -125 microns)

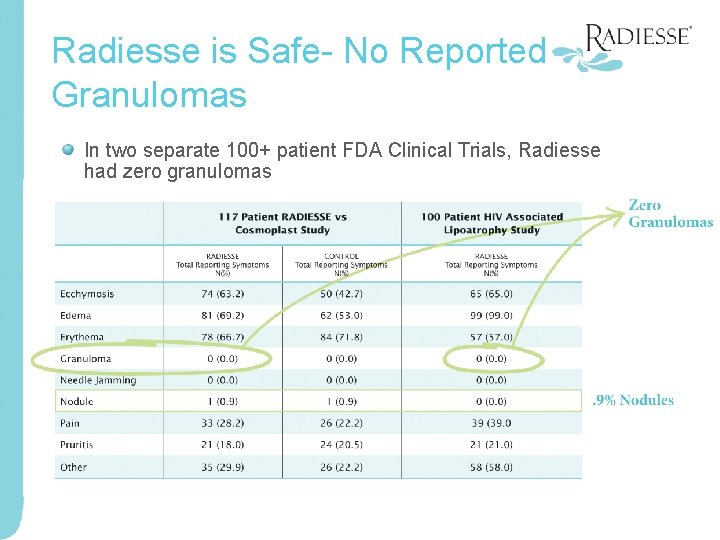
Radiesse is Safe- No Reported Granulomas In two separate 100+ patient FDA Clinical Trials, Radiesse had zero granulomas

Radiesse is Safe- It will not Form Bone in Soft Tissue Bone cannot be formed because: – No osteo-progenitor cells or bone morphogenic proteins are present in the dermis – The Ca. HA present in Radiesse is not bone- it is a mineral component found in bones and teeth Radiesse stimulates the patient’s own collagen to be produced, resulting in a soft and natural appearance and feel The Ca. HA microspheres are synthetically derived and break down naturally over time by the body’s own metabolic processes into Ca 2+ and PO 32+ ions. No ossification or calcification of Ca. HA has been reported in over 6 years of clinical use and >400, 000 syringes injected

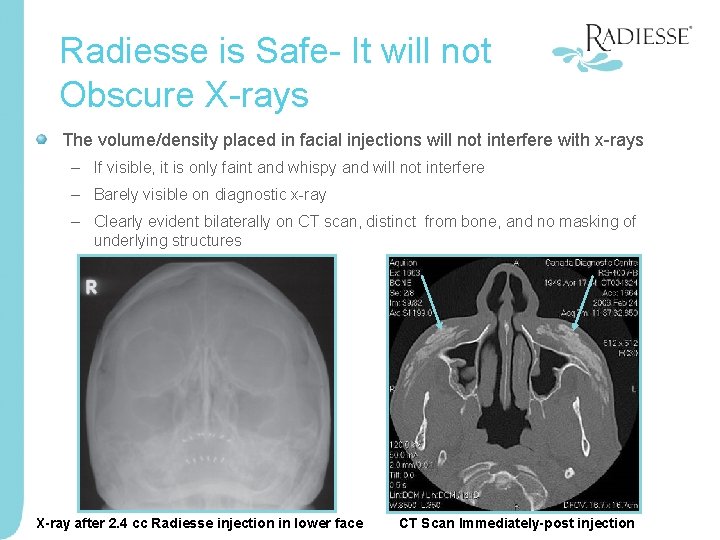
Radiesse is Safe- It will not Obscure X-rays The volume/density placed in facial injections will not interfere with x-rays – If visible, it is only faint and whispy and will not interfere – Barely visible on diagnostic x-ray – Clearly evident bilaterally on CT scan, distinct from bone, and no masking of underlying structures X-ray after 2. 4 cc Radiesse injection in lower face CT Scan Immediately-post injection

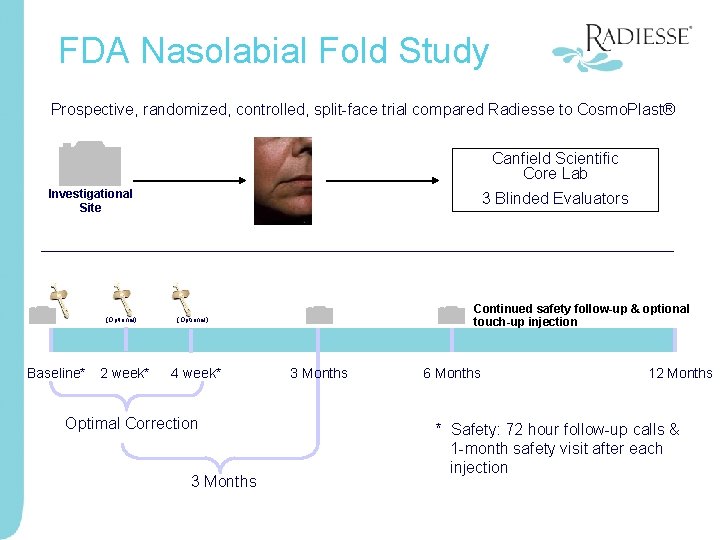
FDA Nasolabial Fold Study Prospective, randomized, controlled, split-face trial compared Radiesse to Cosmo. Plast® Canfield Scientific Core Lab Investigational Site (Optional) Baseline* 2 week* 3 Blinded Evaluators Continued safety follow-up & optional touch-up injection (Optional) 4 week* Optimal Correction 3 Months 6 Months 12 Months * Safety: 72 hour follow-up calls & 1 -month safety visit after each injection

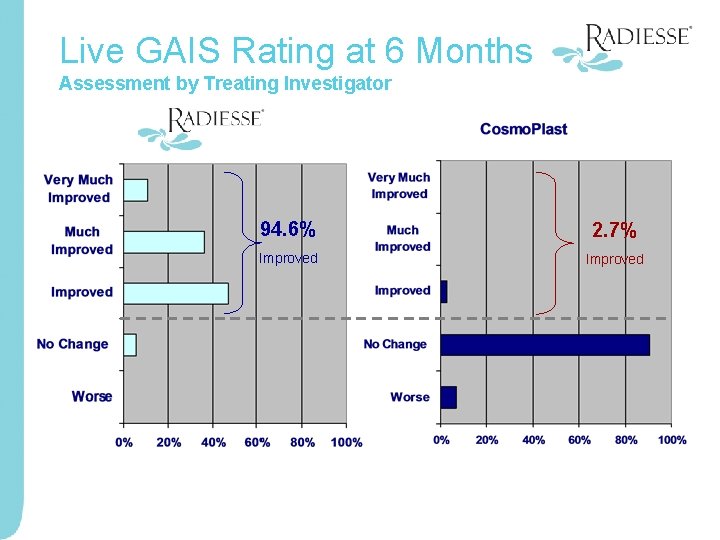
Live GAIS Rating at 6 Months Assessment by Treating Investigator 94. 6% 2. 7% Improved

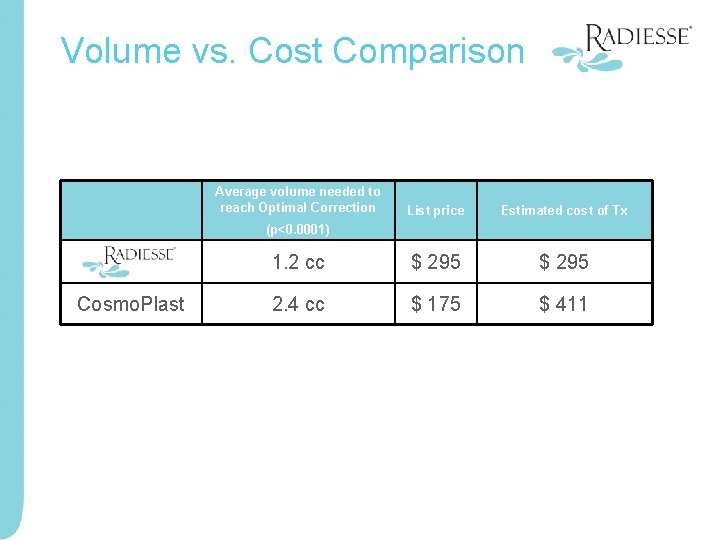
Volume vs. Cost Comparison Average volume needed to reach Optimal Correction List price Estimated cost of Tx (p<0. 0001) Radiesse 1. 2 cc $ 295 Cosmo. Plast 2. 4 cc $ 175 $ 411

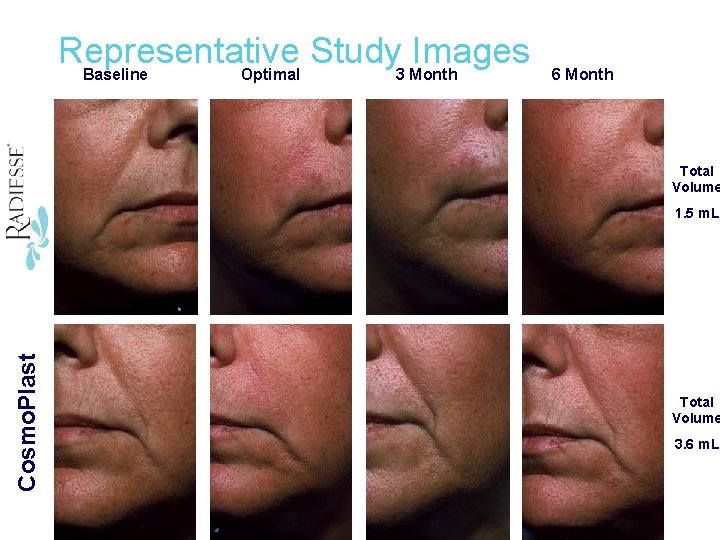
Cosmo. Plast Radiesse Representative Study Images Baseline Optimal 3 Month 6 Month Total Volume 1. 5 m. L Total Volume 3. 6 m. L

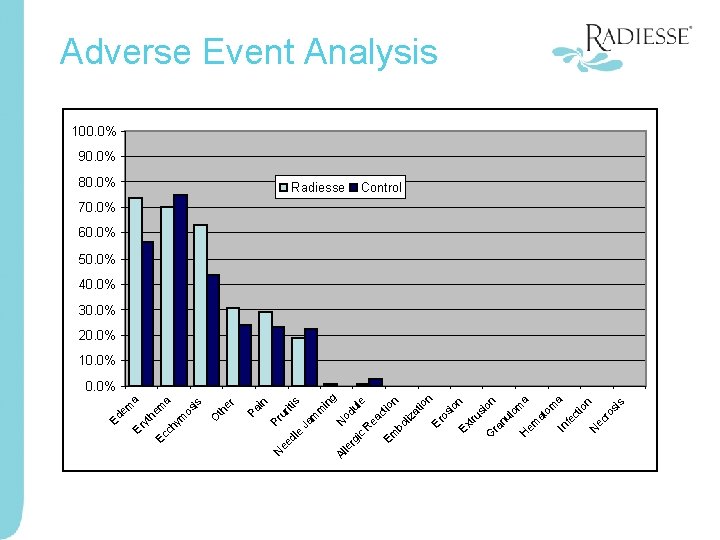
s si n io ro ec N om a ct fe In a om at em H on io n si nu l G ra tru Ex n io at Er os bo liz ct ea R ul e od N m in g s Radiesse Em c rg i le Ja m iti Pr ur 80. 0% Al dl e ee N in Pa O th er is os a a em he m ch ym Ec Er yt Ed Adverse Event Analysis 100. 0% 90. 0% Control 70. 0% 60. 0% 50. 0% 40. 0% 30. 0% 20. 0% 10. 0%

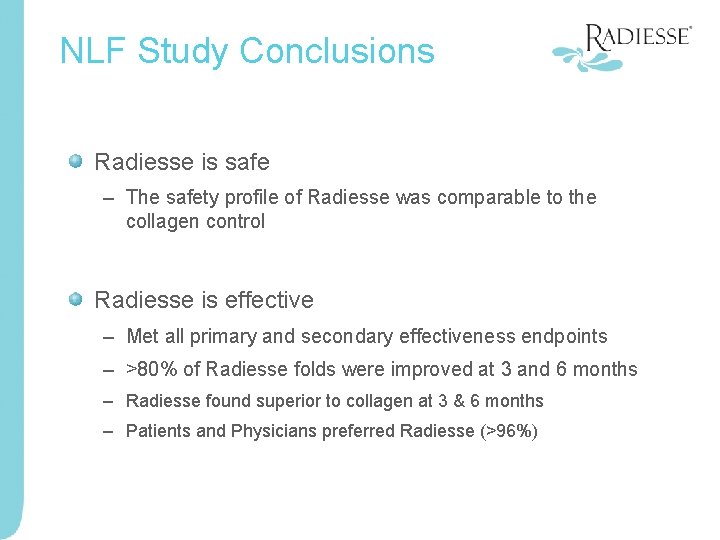
NLF Study Conclusions Radiesse is safe – The safety profile of Radiesse was comparable to the collagen control Radiesse is effective – Met all primary and secondary effectiveness endpoints – >80% of Radiesse folds were improved at 3 and 6 months – Radiesse found superior to collagen at 3 & 6 months – Patients and Physicians preferred Radiesse (>96%)

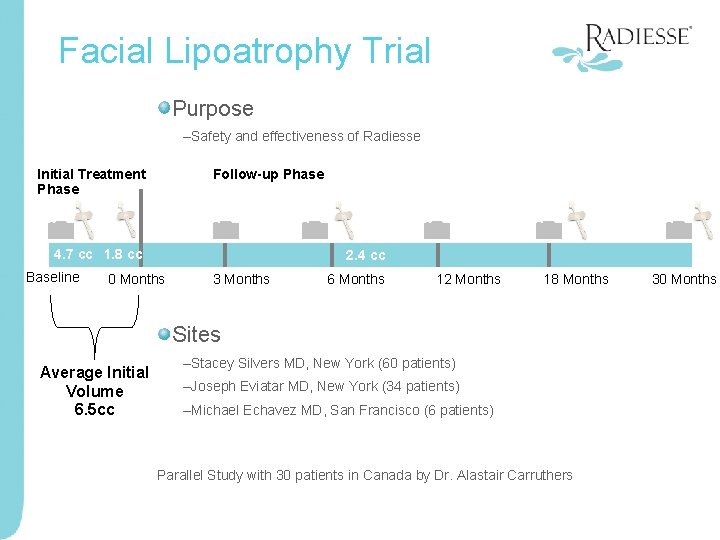
Facial Lipoatrophy Trial Purpose –Safety and effectiveness of Radiesse Initial Treatment Phase Follow-up Phase 4. 7 cc 1. 8 cc Baseline 2. 4 cc 0 Months 3 Months 6 Months 12 Months 18 Months Sites Average Initial Volume 6. 5 cc –Stacey Silvers MD, New York (60 patients) –Joseph Eviatar MD, New York (34 patients) –Michael Echavez MD, San Francisco (6 patients) Parallel Study with 30 patients in Canada by Dr. Alastair Carruthers 30 Months

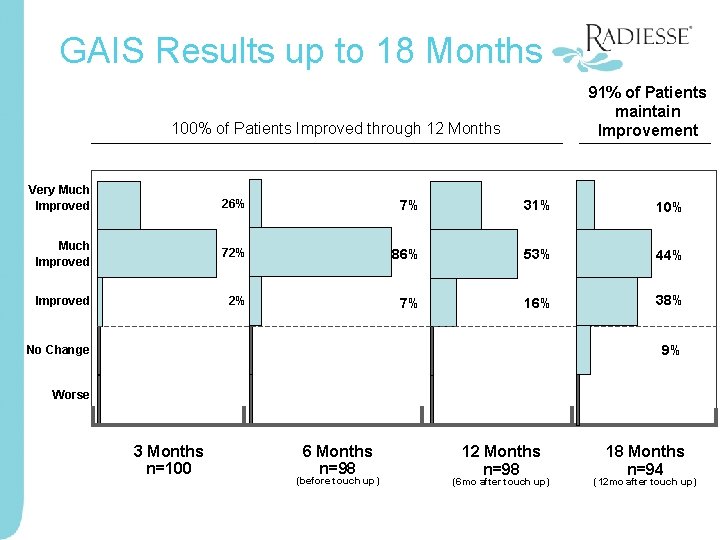
GAIS Results up to 18 Months 91% of Patients maintain Improvement 100% of Patients Improved through 12 Months Very Much Improved 26% 7% 31% 10% Much Improved 72% 86% 53% 44% Improved 2% 7% 16% 38% No Change -- -- Worse -- -3 Months n=100 6 Months n=98 (before touch up) 12 Months n=98 (6 mo after touch up) 9% -18 Months n=94 (12 mo after touch up)

Facial Lipoatrophy Trial Safety Summary No nodules or granulomas at large injection volumes No product related serious adverse events in the study Post-treatment expectations – Pain on injection, resolves quickly – Transient conditions resolving in a few days • Redness • Swelling • Itching • Bruising

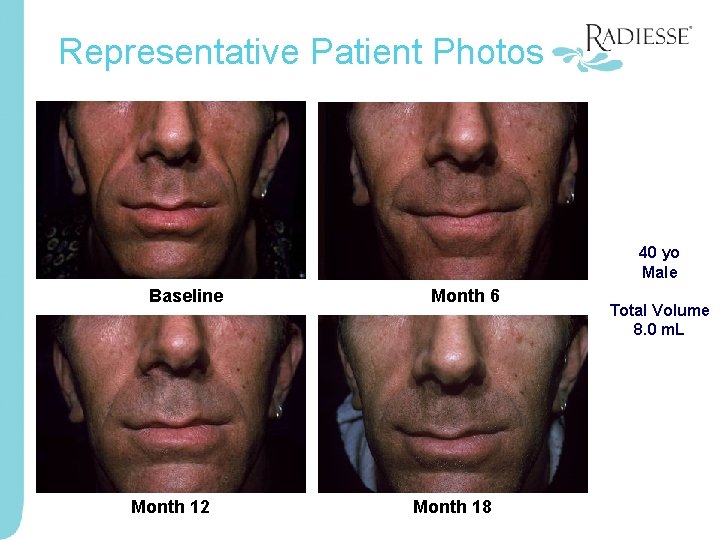
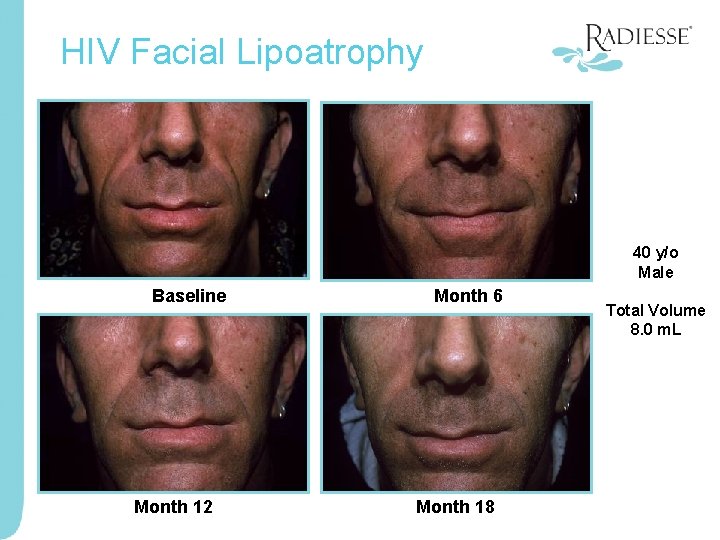
Representative Patient Photos 40 yo Male Baseline Month 12 Month 6 Month 18 Total Volume 8. 0 m. L

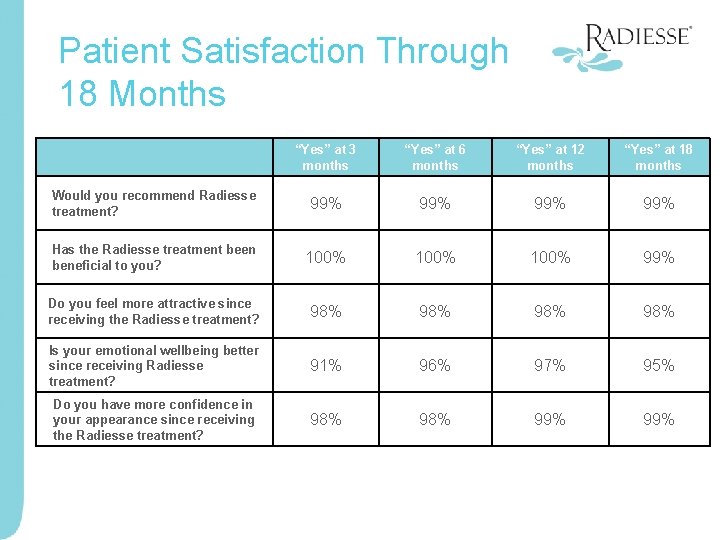
Patient Satisfaction Through 18 Months “Yes” at 3 months “Yes” at 6 months “Yes” at 12 months “Yes” at 18 months Would you recommend Radiesse treatment? 99% 99% Has the Radiesse treatment been beneficial to you? 100% 99% Do you feel more attractive since receiving the Radiesse treatment? 98% 98% Is your emotional wellbeing better since receiving Radiesse treatment? 91% 96% 97% 95% Do you have more confidence in your appearance since receiving the Radiesse treatment? 98% 99%

Radiesse HIV Study Conclusions Radiesse is safe – No reports of serious adverse events – Minor AE’s resolved within 7 days Radiesse is effective – 100% of patients were improved through 12 months – > 90% of patients were improved through 18 months – All primary and secondary endpoints were met – > 90% patient satisfaction through 18 months

Facial Beauty

Facial Aging

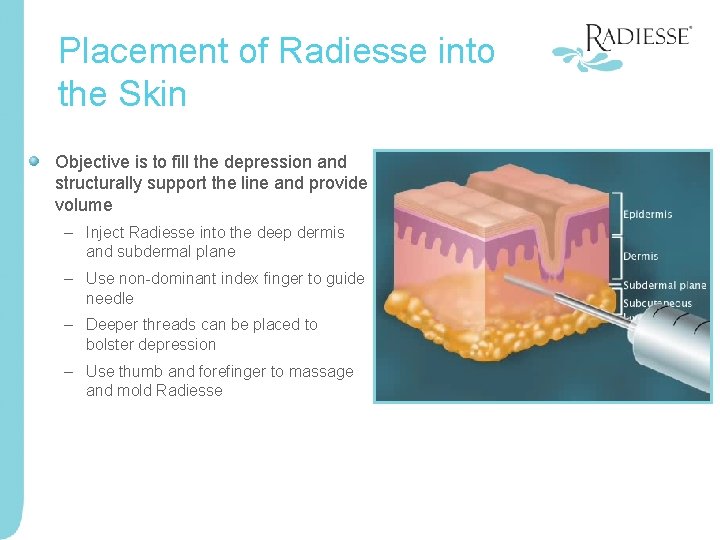
Placement of Radiesse into the Skin Objective is to fill the depression and structurally support the line and provide volume – Inject Radiesse into the deep dermis and subdermal plane – Use non-dominant index finger to guide needle – Deeper threads can be placed to bolster depression – Use thumb and forefinger to massage and mold Radiesse

Pearls of Wisdom for Injecting Radiesse Mark areas to be treated (if necessary) with patient in upright position Make the patient comfortable by providing some level of anesthesia 27 -gauge needle 1. 25”, 1. 5” or 0. 5” needle can be used (area dependent) Inject small amounts (0. 05 cc) in multiple passes Limit puncture sites and stop injecting prior to withdraw of the needle Massage and mold area for desired look Progressively bring the patient to full correction -no overcorrection necessary

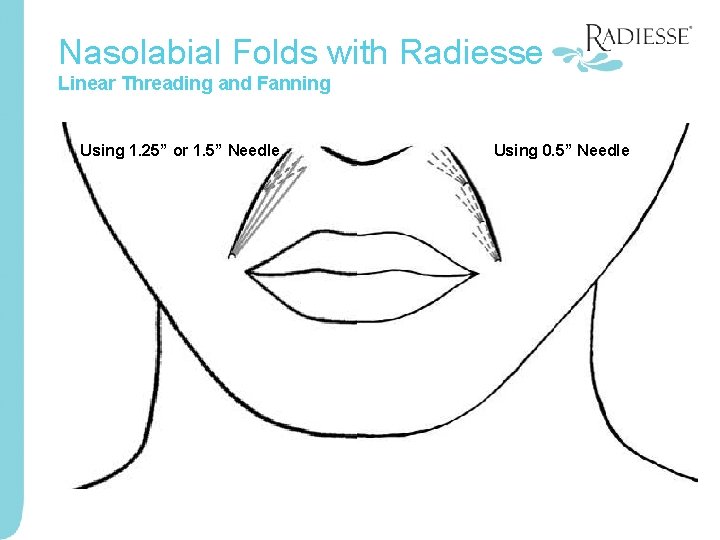
Nasolabial Folds with Radiesse Linear Threading and Fanning Using 1. 25” or 1. 5” Needle Using 0. 5” Needle

Nasolabial Folds with Radiesse Linear Threading and Fanning

Nasolabial Folds with Radiesse Linear Threading and Fanning

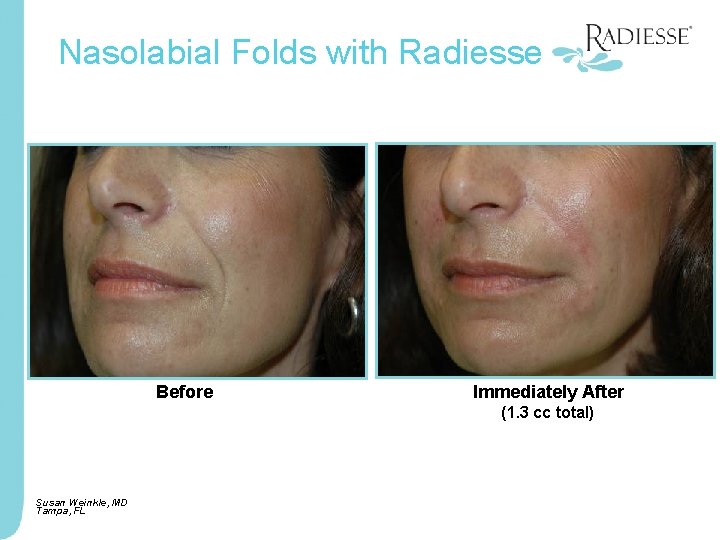
Nasolabial Folds with Radiesse Before Immediately After (1. 3 cc total) Susan Weinkle, MD Tampa, FL

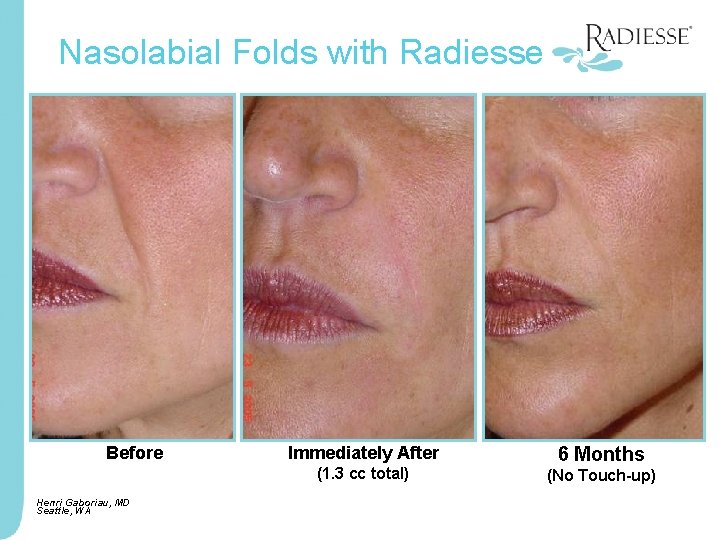
Nasolabial Folds with Radiesse Before Immediately After (1. 3 cc total) Henri Gaboriau, MD Seattle, WA 6 Months (No Touch-up)

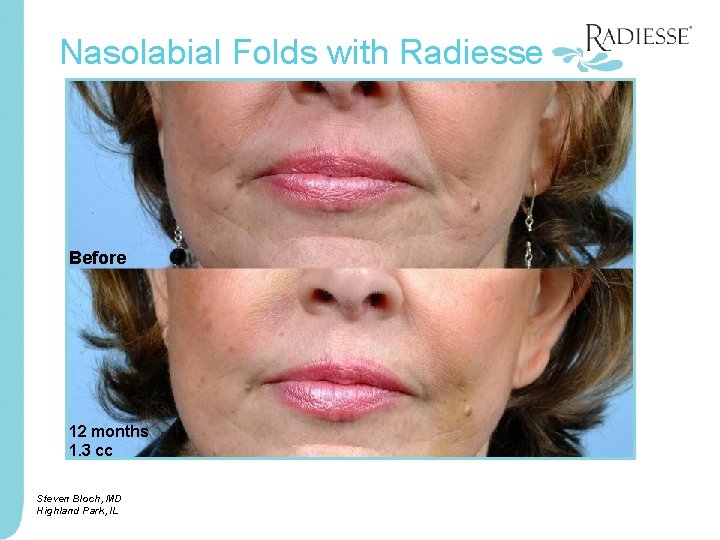
Nasolabial Folds with Radiesse Before 12 months 1. 3 cc Steven Bloch, MD Highland Park, IL

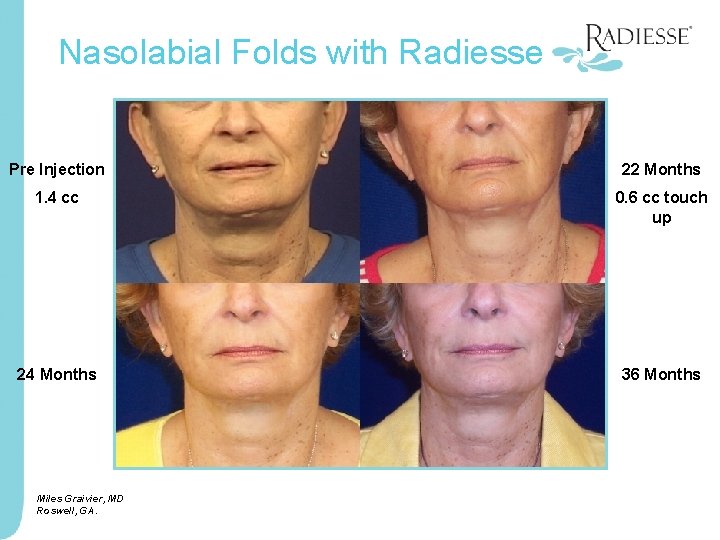
Nasolabial Folds with Radiesse Pre Injection 22 Months 1. 4 cc 0. 6 cc touch up 24 Months 36 Months Miles Graivier, MD Roswell, GA.

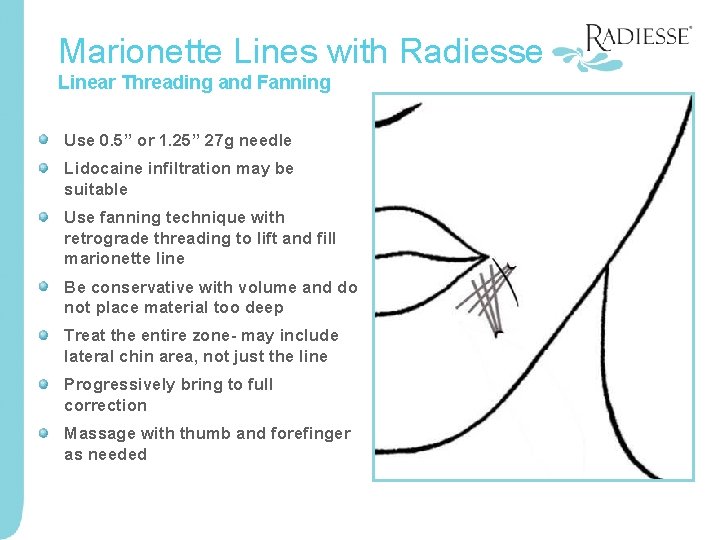
Marionette Lines with Radiesse Linear Threading and Fanning Use 0. 5” or 1. 25” 27 g needle Lidocaine infiltration may be suitable Use fanning technique with retrograde threading to lift and fill marionette line Be conservative with volume and do not place material too deep Treat the entire zone- may include lateral chin area, not just the line Progressively bring to full correction Massage with thumb and forefinger as needed

Marionette Lines with Radiesse Linear Threading and Fanning

Marionette Lines with Radiesse Linear Threading and Fanning

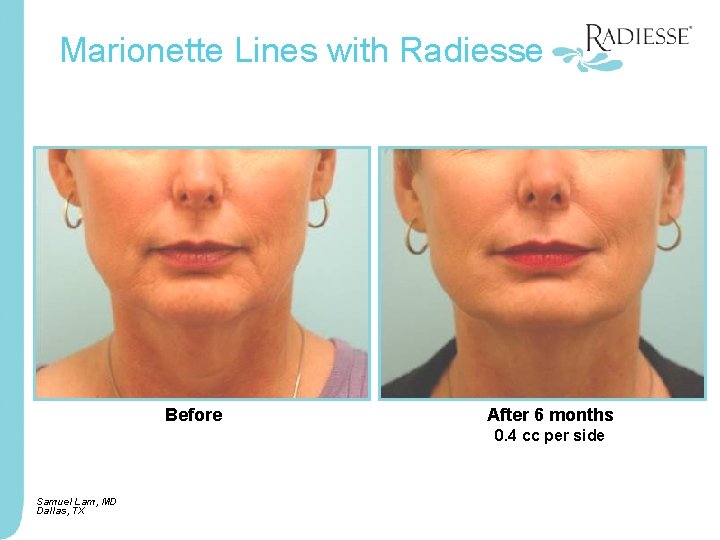
Marionette Lines with Radiesse Before After 6 months 0. 4 cc per side Samuel Lam, MD Dallas, TX

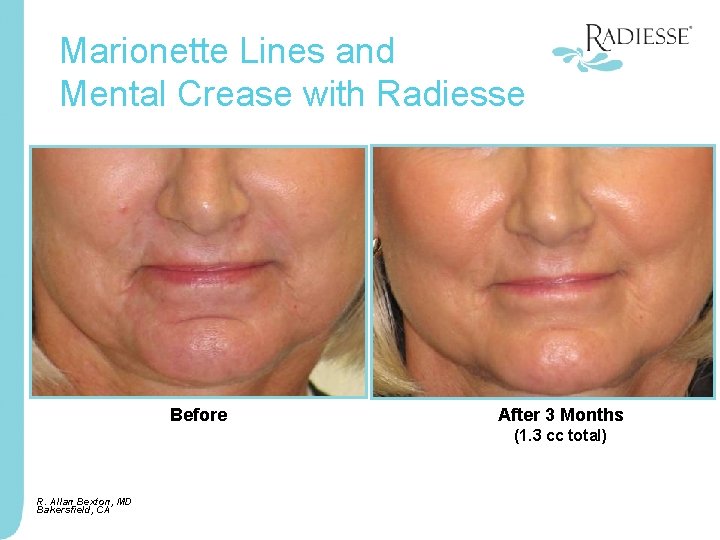
Marionette Lines and Mental Crease with Radiesse Before After 3 Months (1. 3 cc total) R. Allan Bexton, MD Bakersfield, CA

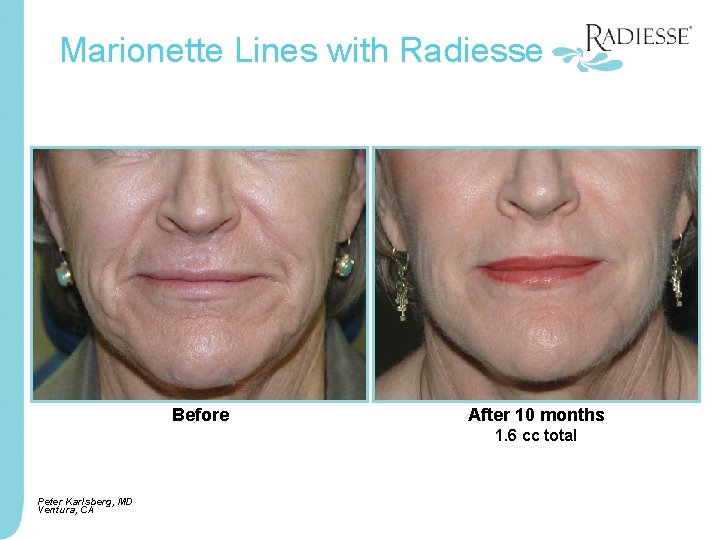
Marionette Lines with Radiesse Before After 10 months 1. 6 cc total Peter Karlsberg, MD Ventura, CA

Pre-jowl Sulcus Technique Linear Threading

Pre-jowl Sulcus Technique Linear Threading

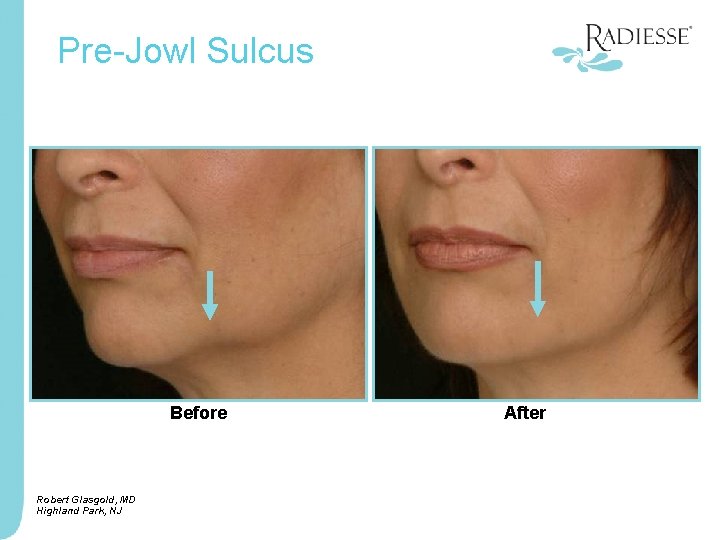
Pre-Jowl Sulcus Before Robert Glasgold, MD Highland Park, NJ After

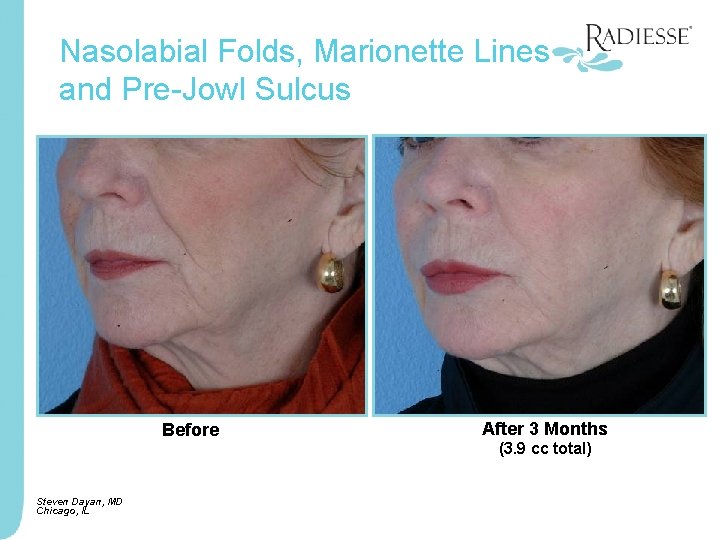
Nasolabial Folds, Marionette Lines and Pre-Jowl Sulcus Before Steven Dayan, MD Chicago, IL After 3 Months (3. 9 cc total)

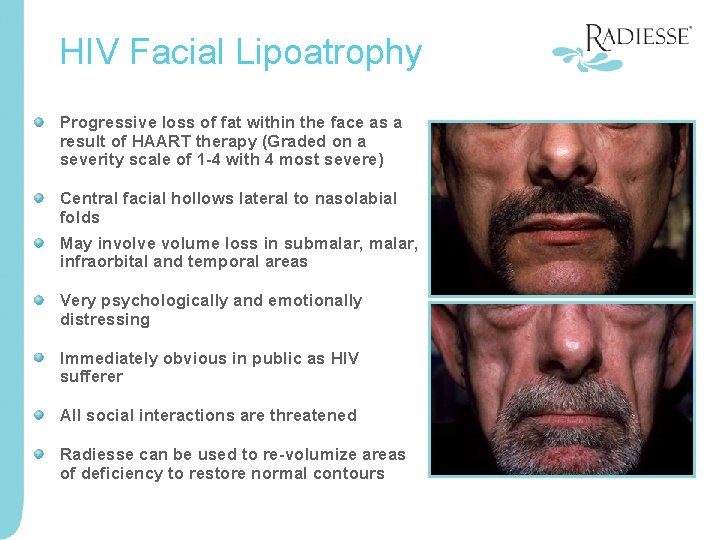
HIV Facial Lipoatrophy Progressive loss of fat within the face as a result of HAART therapy (Graded on a severity scale of 1 -4 with 4 most severe) Central facial hollows lateral to nasolabial folds May involve volume loss in submalar, infraorbital and temporal areas Very psychologically and emotionally distressing Immediately obvious in public as HIV sufferer All social interactions are threatened Radiesse can be used to re-volumize areas of deficiency to restore normal contours

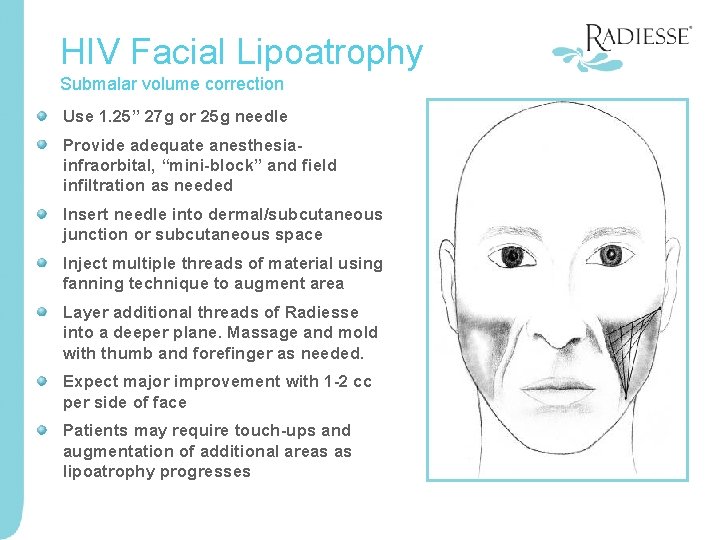
HIV Facial Lipoatrophy Submalar volume correction Use 1. 25” 27 g or 25 g needle Provide adequate anesthesiainfraorbital, “mini-block” and field infiltration as needed Insert needle into dermal/subcutaneous junction or subcutaneous space Inject multiple threads of material using fanning technique to augment area Layer additional threads of Radiesse into a deeper plane. Massage and mold with thumb and forefinger as needed. Expect major improvement with 1 -2 cc per side of face Patients may require touch-ups and augmentation of additional areas as lipoatrophy progresses

HIV Facial Lipoatrophy Submalar volume correction

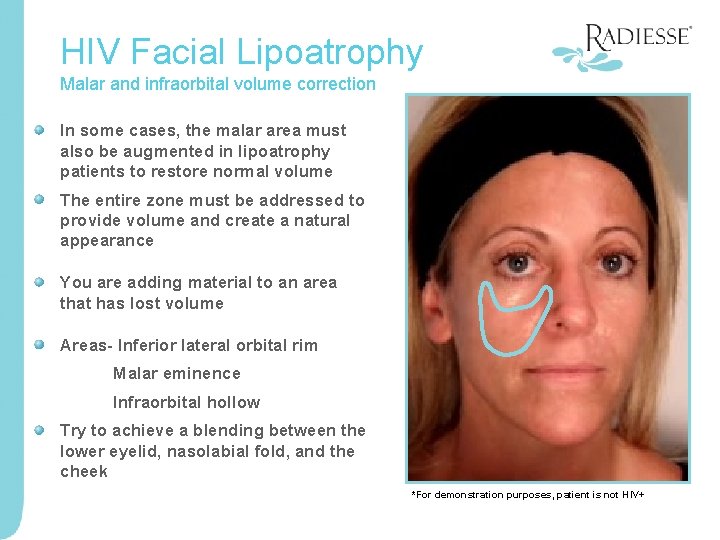
HIV Facial Lipoatrophy Malar and infraorbital volume correction In some cases, the malar area must also be augmented in lipoatrophy patients to restore normal volume The entire zone must be addressed to provide volume and create a natural appearance You are adding material to an area that has lost volume Areas- Inferior lateral orbital rim Malar eminence Infraorbital hollow Try to achieve a blending between the lower eyelid, nasolabial fold, and the cheek *For demonstration purposes, patient is not HIV+

HIV Facial Lipoatrophy Malar and infraorbital volume correction

HIV Facial Lipoatrophy Malar, submalar and infraorbital volume correction

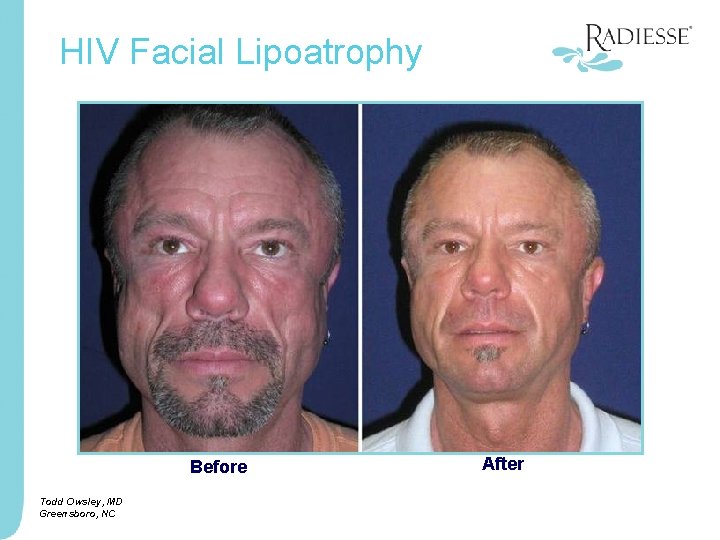
HIV Facial Lipoatrophy Before Todd Owsley, MD Greensboro, NC After Courtesy of, Todd Owsley, MD Greensboro, NC

HIV Facial Lipoatrophy 40 y/o Male Baseline Month 12 Month 6 Month 18 Total Volume 8. 0 m. L

HIV Facial Lipoatrophy Immediate outcome : improvement immediate Provides augmentation lasting a year or more in most patients Swelling is to be expected, but resolves within hours Adverse event profile is minor and similar to other injectables The firmness of the injected material will soften by 2 wks and it will become like the resident soft tissue The patients are ecstatic with the immediate result and this contributes to a positive quality of life

Summary Radiesse is clinically proven to be safe. – It has been used on hundreds of thousands of patients worldwide – Zero granulomas in two separate 100+ patient FDA trials Radiesse provides long lasting correction- about a year or more in most patients Radiesse both fills and stimulates the body to produce new collagen with Calcium-based microspheres Radiesse will grow your aesthetics practice with a wider range of applications Radiesse is a better patient value – Greater patient satisfaction means more patients for you