Physical vs Chemical Properties of Matter Extensive Properties



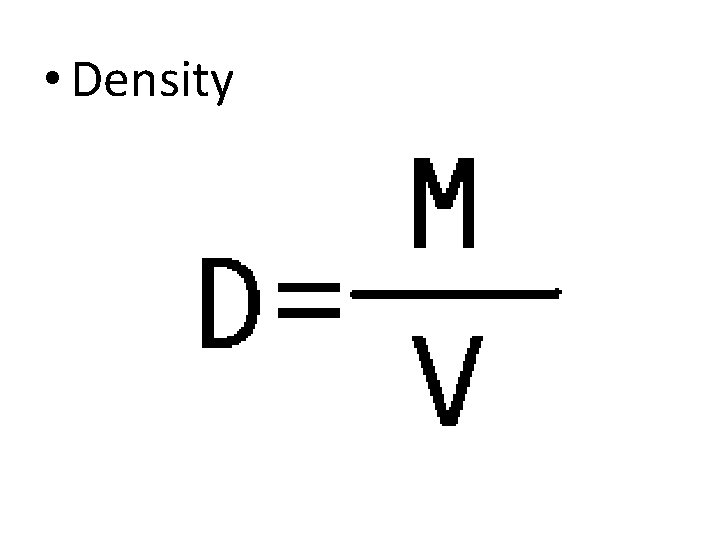




- Slides: 17

Physical vs Chemical Properties of Matter

Extensive Properties of Matter – Extensive - Properties that do depend on the amount of matter present. • Mass - A measurement of the amount of matter in a object (grams). • Weight - A measurement of the gravitational force of attraction of the earth acting on an object. • Volume - A measurement of the amount of space a substance occupies. • Length

Intensive Properties – Intensive - Properties that do not depend on the amount of the matter present. • Color • Odor • Luster - How shiny a substance is. • Malleability - The ability of a substance to be beaten into thin sheets. • Ductility - The ability of a substance to be drawn into thin wires. • Conductivity - The ability of a substance to allow the flow of energy or electricity. • Hardness - How easily a substance can be scratched. • Melting/Freezing Point - The temperature at which the solid and liquid phases of a substance are in equilibrium at atmospheric pressure. • Boiling Point - The temperature at which the vapor pressure of a liquid is equal to the pressure on the liquid (generally atmospheric pressure). • Density - The mass of a substance divided by its volume

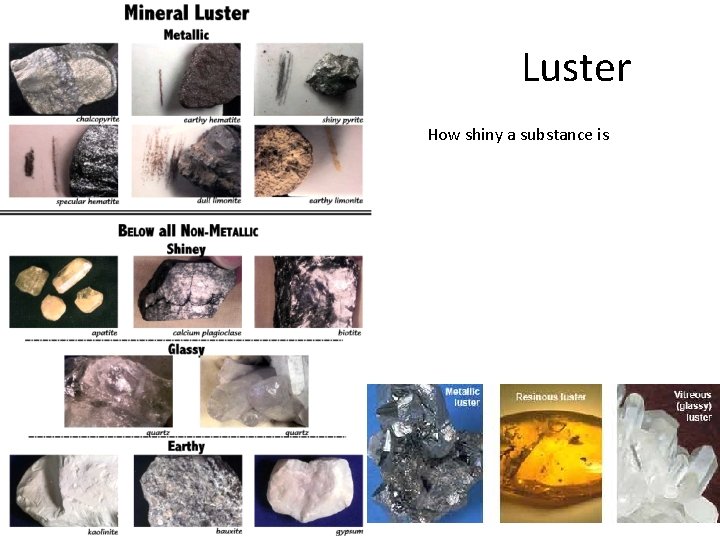
Luster How shiny a substance is

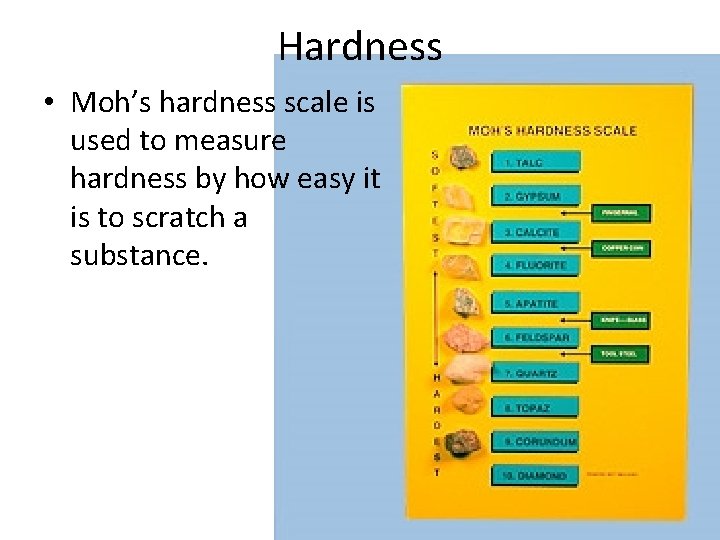
Hardness • Moh’s hardness scale is used to measure hardness by how easy it is to scratch a substance.

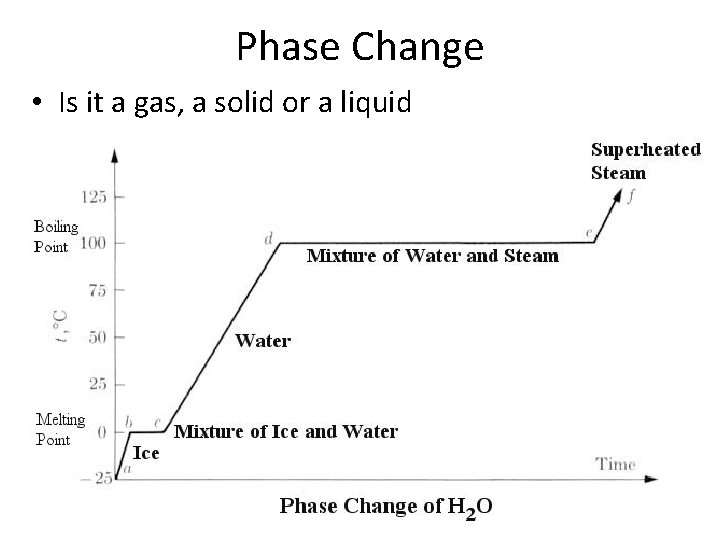
Phase Change • Is it a gas, a solid or a liquid
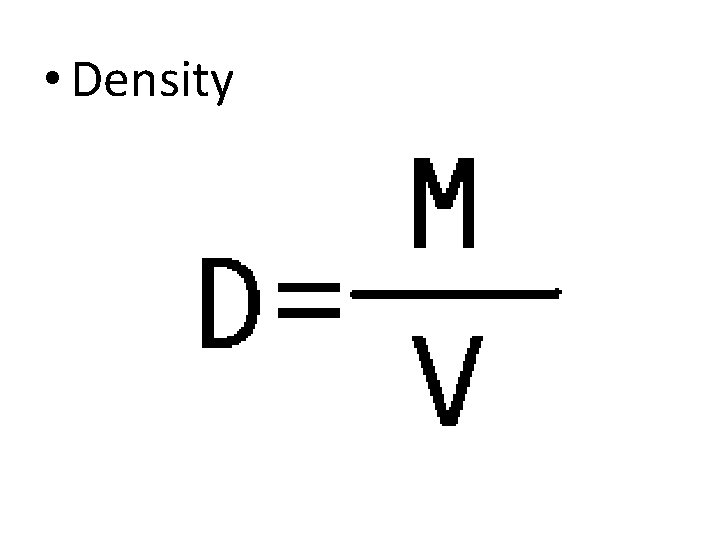
• Density

Chemical Properties of Matter • Chemical Properties of matter deal with how the substances reacts with other substances

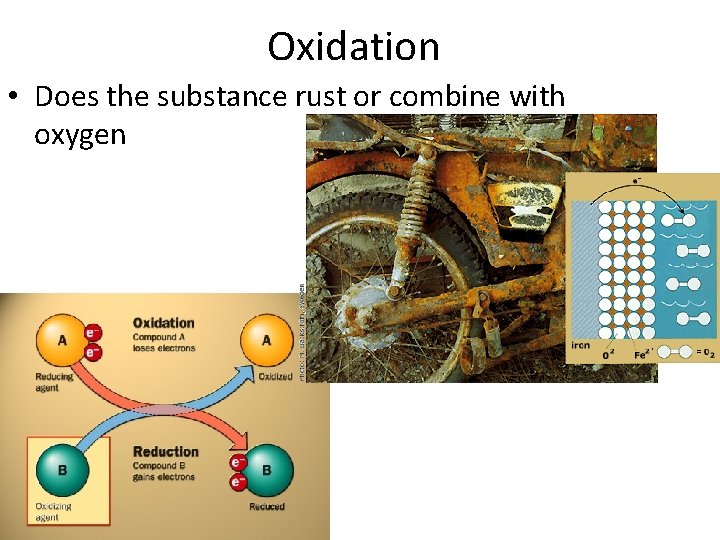
Oxidation • Does the substance rust or combine with oxygen

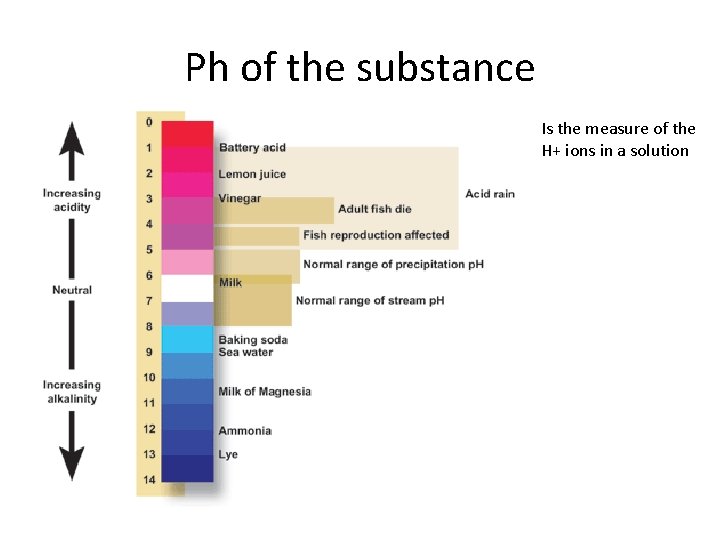
Ph of the substance Is the measure of the H+ ions in a solution

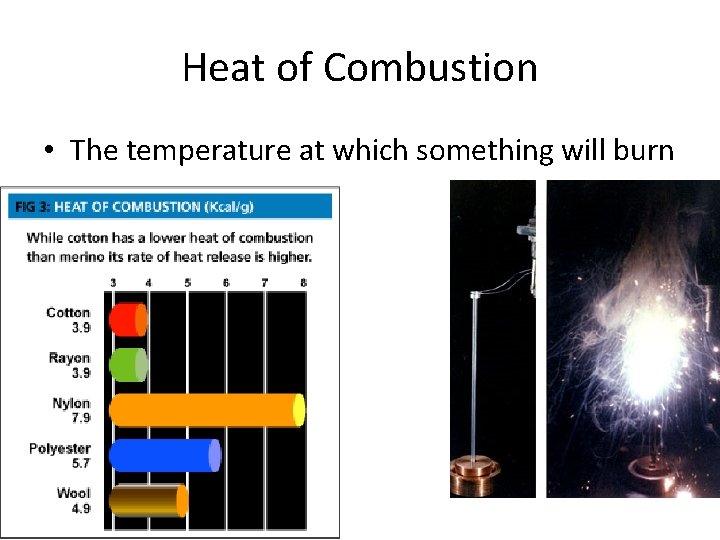
Heat of Combustion • The temperature at which something will burn

Physical Change vs Chemical Change • There are two possible definitions for Physical and Chemical changes: 1. A physical change is reversible, a chemical change is not. – For example, the freezing of water would be a physical change because it can be reversed – the burning of wood is a chemical change - you can't 'unburn' it. 2. A physical change is a change in which no new substance is formed; a chemical change results in the formation of one or more new substances. -Again, consider the previous examples: -Freezing water into ice just results in water molecules which are 'stuck' together - it's still H 2 O. -Burning wood results in ash, carbon dioxide, etc, all new substances which weren't there when you started.


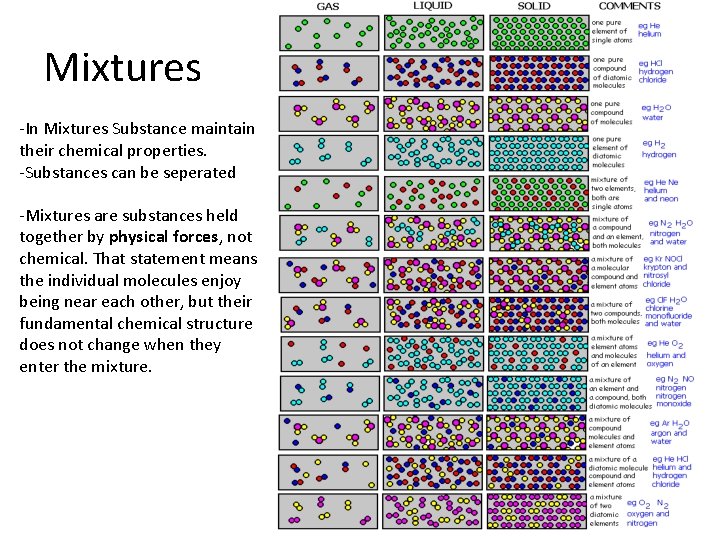
Mixtures -In Mixtures Substance maintain their chemical properties. -Substances can be seperated -Mixtures are substances held together by physical forces, not chemical. That statement means the individual molecules enjoy being near each other, but their fundamental chemical structure does not change when they enter the mixture.

Solutions • Solutions are groups of molecules that are mixed up In a completely even distribution. • example: Sugar in water vs. Sand in water. Sugar dissolves and is spread throughout the glass of water. The sand sinks to the bottom. The sugarwater could be considered a solution. The sand-water is a mixture -A simple solution is basically two substances that are going to be combined. -A solute is the substance to be dissolved (sugar)solvent. -A Solvent is the one doing the dissolving (water). As a The rule of thumb, there is usually more solvent than solut then one solvent

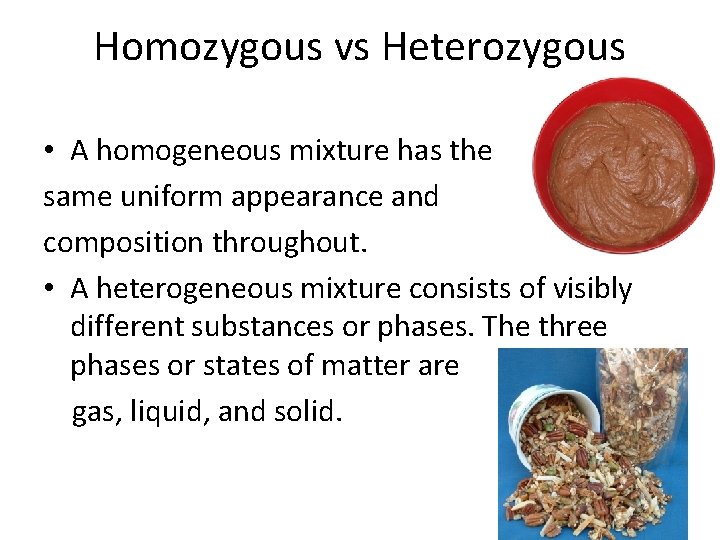
Homozygous vs Heterozygous • A homogeneous mixture has the same uniform appearance and composition throughout. • A heterogeneous mixture consists of visibly different substances or phases. The three phases or states of matter are gas, liquid, and solid.

Different types • Alloys- Metal Mixtures • Colloids- larger particles in a hoogeneous suspension • Suspension- Large particles floating in a solution lifted by flow or motion
 Descriptive matter
Descriptive matter Physical properties of ice cube
Physical properties of ice cube True or false: chemical and physical changes alter matter.
True or false: chemical and physical changes alter matter. Measurable properties of matter examples
Measurable properties of matter examples Chemical properties of matter
Chemical properties of matter Physical properties of matter jeopardy
Physical properties of matter jeopardy Graphic organizer matter classifications
Graphic organizer matter classifications Physical properties of matter
Physical properties of matter Intensive property in thermodynamics
Intensive property in thermodynamics Substance examples
Substance examples Physical properties of sulphuric acid
Physical properties of sulphuric acid A scientist performs an experiment, and an actor performs a
A scientist performs an experiment, and an actor performs a Ideal properties of dental materials
Ideal properties of dental materials Endothermic reaction characteristics
Endothermic reaction characteristics Chemical and physical properties of helium
Chemical and physical properties of helium Physical and chemical properties of boron
Physical and chemical properties of boron Physical/chemical changes & properties color by number
Physical/chemical changes & properties color by number Physical and chemical properties interactive
Physical and chemical properties interactive